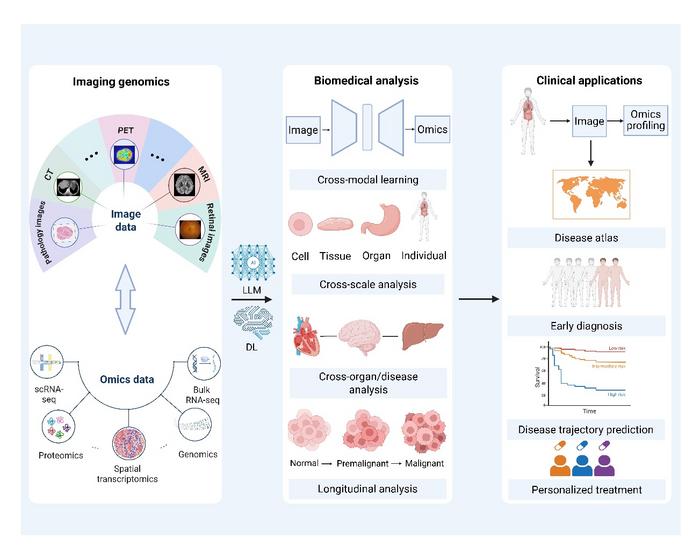
In the rapidly evolving landscape of biomedical research, imaging genomics stands at the frontier, poised to revolutionize our understanding of disease mechanisms and transform clinical practice. Also known as radiogenomics, this interdisciplinary field bridges medical imaging and genomics, enabling the extraction of meaningful correlations between clinical imaging data and the underlying molecular signatures encoded in human genetic material. Despite the remarkable progress in human genomics over the past decade, the phenotypic and clinical implications of many genomic variations remain elusive. Imaging genomics seeks to address this challenge by integrating diverse datasets to uncover the biological and clinical relevance of genomic features.
Recent technological advances have expanded the horizons of imaging genomics beyond traditional single-modality approaches. Modern investigations harness multi-modal imaging data—including computed tomography (CT), magnetic resonance imaging (MRI), X-rays, and ultrasound—alongside a spectrum of molecular data, spanning genomics, transcriptomics, and proteomics. This integrative approach offers unprecedented insights into the molecular architecture of diseases, particularly across complex pathologies such as cancer and cardiovascular disorders. By superimposing imaging phenotypes with multi-omic molecular profiles, researchers are beginning to unravel the pathophysiological mechanisms at a resolution previously unattainable.
Central to the future trajectory of imaging genomics is the advancement of computational frameworks. The past few years have witnessed the emergence of large-scale foundational models, leveraging deep learning architectures and increasingly powerful computational resources. These models show exceptional promise in decoding the high-dimensional, multimodal data intrinsic to imaging genomics. Nonetheless, a major technical bottleneck remains: the absence of a robust, unified foundation model capable of seamlessly integrating cross-scale imaging and omics information. Challenges include harmonizing data across disparately scaled modalities, achieving interpretability in cross-modal analyses, and meeting the formidable demands on computing power.
.adsslot_hlg6f9PFzj{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_hlg6f9PFzj{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_hlg6f9PFzj{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
One of the most exciting frontiers lies in the union of imaging genomics with precision medicine. Imaging genomics complements the phenotypic limitations inherent in electronic medical records by providing detailed molecular and structural disease characterizations. However, current clinical translation efforts are hampered by several factors. Most studies rely on retrospective, cross-sectional data, lacking the longitudinal dimension necessary for tracking disease progression and therapeutic response over time. Furthermore, existing analyses predominantly validate known diagnostic or treatment paradigms, rather than discovering novel biomarkers and therapeutic targets through integrative image-omics correlations.
Recent advancements in cross-modal translation techniques create a paradigm shift in how imaging data might inform omic profiles and vice versa. This burgeoning cross-talk facilitates not only the identification of prognostic biomarkers but also the rational design of targeted therapies. A systematic framework encompassing cross-organ and cross-disease associations stands to radically enhance our understanding of disease etiology. By embedding principles of biological connectivity and multi-organ pathophysiological pathways, imaging genomics is positioned to provide comprehensive disease atlases that elucidate early disease onset, progression trajectories, and multisystem interactions.
The roadmap for the coming decade envisions a transformative shift in imaging genomics from retrospective data validation to integrative systems biology modeling. Such modeling paradigms will utilize interpretative deep learning and large language models to generate interpretable, multimodal disease representations. These will underpin biomarker discovery and the identification of novel therapeutic targets, ultimately empowering clinicians to deliver precise, individualized medical interventions. The integration of these technologies promises to bridge the conceptual gap between molecular biology and clinical applicability.
As Dr. Xiao Ping Cen from the University of Chinese Academy of Sciences highlights, the evolution of imaging genomics will elevate the field from isolated correlation studies to holistic systems-level insights. The increased accessibility to global data collaboration networks, combined with advances in artificial intelligence, positions imaging genomics as a cornerstone of future diagnosis and treatment. These innovations are expected to culminate in clinical decision-making tools capable of tailoring therapy plans to the unique genetic and phenotypic profiles of each patient.
The challenges inherent in this transition are non-trivial. New algorithms must overcome the complexities inherent to multi-modality data heterogeneity, as well as the interpretability crisis characteristic of many “black-box” AI models. Additionally, computational infrastructures will need to scale efficiently to manage the massive datasets generated by high-throughput sequencing and advanced imaging platforms. Researchers increasingly emphasize model transparency and explainability to foster clinical trust and regulatory acceptance.
A significant portion of future research will also focus on longitudinal data integration. By capturing temporal changes in imaging and omic profiles, scientists can delineate disease progression pathways, identify early markers of therapeutic resistance, and optimize intervention timing. The incorporation of longitudinal analyses introduces dynamic modeling capabilities that can predict future outcomes and simulate intervention effects, advancing imaging genomics beyond static snapshots to predictive, real-world clinical utility.
The broad applicability of imaging genomics extends across diverse disease frameworks. In oncology, the correlation of tumor imaging phenotypes with mutational landscapes paves the way for non-invasive tumor characterization and personalized treatment planning. In cardiovascular medicine, imaging-genomic associations promise improved stratification of atherosclerotic risk and tailored management protocols. The integration of multi-organ data sets enables holistic patient profiling, accounting for systemic factors influencing disease manifestation and treatment response.
Ultimately, imaging genomics encapsulates the synergistic potential of advanced imaging technologies, high-throughput omics, and state-of-the-art artificial intelligence methods. As we progress into an era defined by precision medicine, the capacity to interpret complex biological data within a unified, clinically actionable framework becomes paramount. The burgeoning field of imaging genomics offers a visionary path forward—a confluence where biology, technology, and medicine coalesce to drive transformative healthcare outcomes.
Subject of Research: Imaging Genomics and Multimodal Data Integration in Precision Medicine
Article Title: Roadmap for Imaging Genomics in the Next Decade
Web References:
http://dx.doi.org/10.1016/j.scib.2025.04.058
Image Credits: Created with Advanced Deep Learning and Large Language Models Frameworks
Keywords: Imaging Genomics, Radiogenomics, Deep Learning, Large Language Models, Multimodal Data Integration, Precision Medicine, Biomarker Discovery, Systems Biology, Cross-Modal Analysis, Disease Atlas
Tags: advancements in medical imaging technologyAI in precision medicinecardiovascular disorders and genomics integrationcomputational frameworks in biomedical researchcross-modal approaches in disease analysisfuture of personalized medicine with AI.imaging genomics for disease understandinginsights into cancer pathology through imagingintegrating imaging and genomic datamolecular signatures in human geneticsmulti-modal imaging techniques in healthcareradiogenomics and clinical applications