In the quest to develop cancer therapies that minimize harm to healthy tissues, a groundbreaking approach employing ultrasound to chemically activate prodrugs inside tumors has emerged from the laboratories of the Chinese Academy of Sciences. Traditional chemotherapy, although effective against malignant cells, often results in widespread cytotoxicity that damages healthy tissues and causes debilitating side effects. The newly developed method showcases an innovative strategy to overcome these limitations by precisely controlling drug activation within the tumor microenvironment through non-invasive ultrasound energy.
Conventional prodrugs remain inactive until triggered by specific biochemical cues within the tumor, such as low pH or tumor-associated enzymes. However, these intrinsic tumor characteristics are highly heterogeneous and poorly controlled, limiting the efficacy and consistency of prodrug activation in clinical scenarios. Researchers have explored numerous external triggers—light, heat, magnetic fields—to enhance prodrug activation, but penetrating deep tissue with sufficient precision and safety remains a formidable challenge. Ultrasound offers a compelling alternative due to its ability to focus energy non-invasively at substantial depths, which is routinely leveraged in diagnostic imaging.
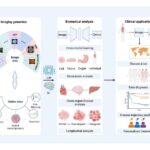
The team, based at the Changchun Institute of Applied Chemistry alongside collaborators from the University of Science and Technology of China and Jilin University, conceptualized a system wherein prodrug-laden nanoparticles respond to focused ultrasound stimulation, unleashing potent therapeutics precisely within tumors. This system utilizes a prodrug compound, R848-N₃, encapsulated within biocompatible nanoparticles along with a catalytic agent, riboflavin tetrabutyrate, designed to harness ultrasonic energy for chemical activation. Upon ultrasound exposure, the catalyst initiates a reaction that converts the inert prodrug into an active immunostimulatory agent.
.adsslot_cgiufZevHP{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_cgiufZevHP{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_cgiufZevHP{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Crucially, the mechanism exploits the naturally abundant reducing agent nicotinamide adenine dinucleotide (NADH) present within cells to propel the catalytic reaction forward. The riboflavin derivative acts as a photocatalyst analogously but is instead activated by ultrasound energy. This selective activation in the tumor milieu avoids systemic immune stimulation and off-target toxicity, which are major drawbacks of traditional immunotherapy and chemotherapy regimens.
Preclinical trials employing murine models of colon cancer delivered astonishing results, with tumor volume suppression reaching 99% and complete remission achieved in over two-thirds of treated mice. During these trials, non-target tissues exhibited no significant cytotoxicity or inflammatory damage, highlighting the system’s exceptional precision and safety. The nanoparticles’ stability and biocompatibility ensured effective accumulation within solid tumors through enhanced permeability and retention effect, further enhancing treatment specificity.
This ultrasound-triggered prodrug activation represents a paradigm shift from physical disruption of cancer cells by ultrasound, such as via thermal ablation or mechanical cavitation, to precise chemical modulation of therapeutics within the tumor microenvironment. By converting high-frequency sound waves into chemical energy through nanoscale catalysis, the technology pioneers a new class of spatially controlled therapies with potential applications far beyond oncology.
The ramifications of this advance extend into immuno-oncology, as the active drug released promotes immune cell recruitment and activation, effectively turning “cold” tumors into “hot” ones that respond robustly to immunotherapeutic intervention. This synergistic effect could prove transformative in managing cancers traditionally resistant to immune checkpoint inhibitors or other modern immunotherapies, expanding the therapeutic arsenal available to clinicians.
From a materials science perspective, the rational design of the nanoparticle carrier and catalyst assembly is vital to achieving this breakthrough. The team carefully engineered biocompatible polymers that shield the prodrug and catalyst during systemic circulation but open in response to ultrasound-triggered catalytic activity. The modularity of this platform also allows for tailoring to different prodrugs and catalysts, opening avenues for personalized medicine based on tumor histology and patient-specific factors.
Safety considerations remain paramount, yet the use of ultrasound circumvents many pitfalls faced by other external triggers. Unlike ultraviolet or visible light, which suffers from limited penetration and potential tissue damage, ultrasonic waves can be focused on deep-seated tumors without invasive procedures or harmful irradiation. Moreover, dosimetry can be precisely controlled to mitigate heating effects and preserve surrounding healthy structures.
The research team’s future plans involve optimizing the nanoparticle formulation for clinical use and progressing toward human trials. Scaling production under good manufacturing practices (GMP) and undertaking comprehensive toxicological assessments will be critical next steps. Should clinical translation prove successful, this approach promises an entirely new therapeutic modality combining the precision of physical stimulation with the potency of targeted chemical drug activation.
Dr. Zhaohui Tang, one of the corresponding authors, emphasized the broader implications of this technology: “Ultrasound has long been confined to imaging and mechanical disruption in medicine. Our findings reveal it as a powerful switch to selectively activate therapies at tumor sites, fundamentally changing how we deliver drugs in vivo.” This sentiment reflects the technology’s potential to revolutionize multiple biomedical fields through intertwining physical stimuli and chemical processes.
In conclusion, this ultrasound-driven prodrug activation marks a significant leap forward in cancer therapy development. By seamlessly integrating nanoscale catalysis, prodrug chemistry, and focused ultrasound, researchers have created a platform capable of precise, safe, and potent tumor eradication. This innovation paves the way not only for more effective cancer treatments but also for novel applications in immunotherapy and nanomedicine, heralding a new era of intelligent, controlled therapeutics.
The study, recently published in the prestigious journal National Science Review, is supported by funding from the National Key R&D Program of China and the National Natural Science Foundation. The collaborative efforts of experts from polymer science, nanotechnology, and biomedical engineering emphasize the multidisciplinary nature essential for such pioneering inventions. As interest in ultrasound-based therapeutic technologies surges, this breakthrough sets a new benchmark, underscoring China’s growing leadership in cutting-edge biomedical research.
Subject of Research: Ultrasound-triggered chemical activation of prodrugs for targeted cancer therapy
Article Title: Ultrasound-responsive nanoparticle system for catalytic prodrug activation in tumor immunotherapy
Web References: 10.1093/nsr/nwaf140
Keywords
Ultrasound therapy, prodrug activation, nanoparticle catalysis, tumor immunotherapy, targeted drug delivery, riboflavin catalyst, NADH, colon cancer, nanomedicine, focused ultrasound, chemotherapy alternative, tumor microenvironment
Tags: Chinese Academy of Sciences researchenhancing prodrug efficacyinnovative cancer treatment strategieslow-intensity ultrasound applicationsminimizing chemotherapy side effectsnanoparticles in drug deliverynon-invasive cancer treatment methodsprecision medicine in oncologyprodrug activation techniquestumor microenvironment targetingultrasound in cancer therapyultrasound-guided drug therapy