For nearly two centuries, inhalational anesthetics have stood as the cornerstone of modern general anesthesia, facilitating countless surgical procedures with remarkable efficacy. Despite their pervasive use and undeniable clinical importance, the precise molecular underpinnings of how these agents induce anesthesia have long remained elusive. Prevailing evidence has suggested that inhalational anesthetics act through multiple protein targets, but many aspects of their mechanisms, including the identification of all target molecules, have yet to be fully decoded. A new landmark study now illuminates a critical missing piece in this complex puzzle, revealing the type 1 ryanodine receptor (RyR1) as a direct and functional target of inhalational anesthetics, advancing our understanding of general anesthesia at the molecular level.
The investigation, spearheaded by Professor Hiroki Ueda and his team at the Graduate School of Medicine, The University of Tokyo, demonstrates that RyR1—a calcium release channel embedded in the membrane of the endoplasmic reticulum—plays a hitherto underappreciated role in mediating the anesthetic effects of isoflurane, a widely used inhalational agent. Clinically, mutations in RyR1 are known to predispose patients to malignant hyperthermia, a rare but potentially fatal condition triggered by certain anesthetics. Yet, until now, the direct molecular interaction between inhalational anesthetics and RyR1 had not been empirically or mechanistically established.
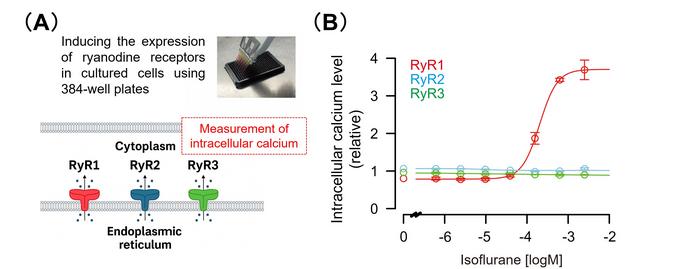
Using an innovative experimental setup capable of directly detecting calcium release events from the endoplasmic reticulum, the researchers first confirmed that isoflurane and other inhalational anesthetics induce the activation of RyR1 channels. This activation promotes the liberation of calcium ions into the cytoplasm, an effect with profound cellular signaling consequences. Through meticulous biochemical and structural analysis, they pinpointed specific amino acid residues within RyR1 that are critical for this anesthetic-induced activation, enabling the delineation of the putative isoflurane binding site on the receptor.
.adsslot_USqRChpDK6{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_USqRChpDK6{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_USqRChpDK6{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
To rigorously test the physiological relevance of this interaction, the team engineered a genetically modified mouse model—known as a knock-in mouse—that carried mutations in RyR1 rendering it unresponsive to isoflurane activation. Intriguingly, when subjected to isoflurane anesthesia, these mutant mice demonstrated a markedly diminished sensitivity compared to their wild-type counterparts. This partial reduction in anesthetic sensitivity strongly supports the conclusion that RyR1 activation is not merely correlative but causally implicated in the induction of anesthesia by isoflurane.
Expanding beyond mechanistic insights, the investigators embarked on a drug discovery effort using in silico compound screening based on the identified isoflurane binding pocket. This computational approach uncovered novel chemical entities capable of modulating RyR1, some of which exhibited sedative-like effects in murine models, thereby underscoring the therapeutic potential of targeting RyR1 for anesthesia or sedation. This not only validates RyR1 as a functional target but also paves the way for the rational design of next-generation anesthetics with improved efficacy and safety profiles.
The implications of these findings extend well beyond fundamental biology. By establishing a direct molecular link between RyR1 activation and the anesthetic state, this research challenges the traditional paradigms that have long dominated anesthesia pharmacology. It raises the prospect that other inhalational anesthetics may share similar interactions with RyR1 or related calcium signaling pathways, thus expanding the landscape of anesthetic pharmacodynamics. Furthermore, the ability to genetically modulate anesthetic sensitivity via RyR1 mutations highlights novel avenues for personalized anesthesia tailored to individual genetic profiles, potentially mitigating risks such as malignant hyperthermia.
At the cellular level, RyR1 serves as a gatekeeper for calcium release from intracellular stores, a process fundamental to muscle contraction, neuronal excitability, and numerous signaling cascades. Calcium ions function as ubiquitous second messengers, influencing processes as diverse as gene transcription and metabolic regulation. Isoflurane-induced RyR1 activation disrupts calcium homeostasis transiently, which may translate into the suppression of neuronal activity central to the induction and maintenance of general anesthesia. This conceptual framework integrates molecular biophysics with neurophysiology, offering a holistic view of anesthetic action.
This pioneering study was conducted within the Ueda Biological Timing Project under the auspices of the Exploratory Research for Advanced Technology (ERATO) program, funded by the Japan Science and Technology Agency (JST). The project’s broader aim to elucidate “biological time” across multiple organizational scales aligns with the discovery of anesthetic mechanisms, which inherently involve temporally coordinated alterations in brain and systemic function. Through rigorous experimentation and interdisciplinary collaboration, this work exemplifies how systems pharmacology can unlock new understanding of complex biomedical phenomena.
From a clinical perspective, these insights provide a conceptual basis for developing safer anesthetic protocols, especially for patients harboring RyR1 mutations associated with malignant hyperthermia susceptibility. The current reliance on dantrolene, an RyR inhibitor, for emergency treatment underscores the critical nature of RyR-mediated calcium dysregulation during anesthesia. By selectively modulating RyR1 activity, future anesthetics might minimize adverse metabolic responses while maximizing anesthetic depth and recovery quality.
Moreover, the study’s methodology—combining electrophysiological assays, molecular biology, genetic engineering, computational chemistry, and behavioral pharmacology—sets a new benchmark for mechanistic research in anesthesiology. The integration of in silico screening, which expedites the identification of RyR1 modulators, represents a powerful strategy to translate basic molecular discoveries into clinically relevant therapeutics. As such, this paradigm may well be adopted to explore other ion channels and receptors implicated in anesthesia and sedation.
Importantly, this discovery challenges the previous notion that anesthetic mechanisms are too complex for single targets to dominate their effects. Rather, it suggests a modular approach, where specific protein targets like RyR1 contribute significantly to the integrated physiological state of anesthesia. Understanding these components at the atomic and cellular levels unlocks new principles for anesthetic pharmacology and potentially revolutionizes drug development paradigms for central nervous system depressants.
The findings reported in the American scientific journal PLOS Biology on June 3, 2025, thus represent a transformative advance in our grasp of anesthesia’s molecular choreography. They not only elevate RyR1 to a central role in anesthesia induction by isoflurane but also herald exciting prospects for improved anesthetic agents engineered with precision targeting. As research continues, such breakthroughs promise to enhance perioperative care and open new frontiers in neuropharmacology and biomedical science.
Subject of Research: Animals
Article Title: Isoflurane activates the type 1 ryanodine receptor to induce anesthesia in mice
News Publication Date: June 3, 2025
Web References: http://dx.doi.org/10.1371/journal.pbio.3003172
Image Credits: Department of Systems Pharmacology, Graduate School of Medicine, UTokyo
Keywords: Inhalational Anesthetics, RyR1, Isoflurane, Calcium Release, Ryanodine Receptor, General Anesthesia, Knock-in Mouse, Malignant Hyperthermia, Endoplasmic Reticulum, Sedative Agents, Molecular Mechanism, Pharmacology
Tags: anesthesia research breakthroughscalcium release channels in anesthesiaclinical implications of RyR1 mutationsendoplasmic reticulum in anesthesiageneral anesthesia advancementsinhalational anesthetics mechanism of actionisoflurane anesthetic effectsmalignant hyperthermia and anestheticsmolecular targets of anesthesiasurgical procedures and anesthesiatype 1 ryanodine receptor RyR1understanding general anesthesia at molecular level