This is a medical illustration of multidrug-resistant, Pseudomonas aeruginosa bacteria [CDC/ Antibiotic Resistance Coordination and Strategy Unit /Jennifer Oosthuizen]
Scientists at the University of Nottingham have discovered how specific surface patterns on plastics used for medical devices can drastically reduce the ability of bacteria to attach and multiply, potentially offering an approach to help prevent infections on medical devices, such as catheters.
The collective findings of the study, which included tests in live mice, showed that when bacterial cells encounter patterned grooves on a surface, they lose their ability to attach and form biofilms. Biofilms are surface associated slime-cities that help protect the bacteria from the body’s natural defenses against infection. Preventing bacterial biofilm formation could in turn prevent infection before it can become fully established and would also positively activate the immune system to get rid of any individual bacteria that were there.
Research co-lead Professor Paul Williams, PhD, in the School of Life Sciences, said, “Our findings could help to reduce the high number of infections in healthcare settings associated with medical devices. Not only could this method prevent bacteria sticking but also activate the body’s immune system to kill any bacteria that have stuck to the surface.”
Williams is senior and co-corresponding author of the researchers’ published paper in Nature Communications, titled “Combinatorial discovery of microtopographical landscapes that resist biofilm formation through quorum sensing mediated autolubrication.” The international study team included Professor Morgan Alexander, PhD, in the School of Pharmacy, and colleagues in the School of Computer Science at the University of Nottingham, together with Jan DeBoer, PhD, at the Department of Biomedical Engineering, Eindhoven University of Technology.
“Bacteria generally grow as individual planktonic cells in suspension or as organized biofilm communities usually attached to a surface,” the authors wrote. Many medical implants, such as catheters and breathing tubes, are made of plastics and are commonplace in hospitals. Once bacteria have stuck to the plastic surface and developed into a biofilm, they can be very difficult to treat with antibiotics. “In a clinical context, bacterial biofilm formation on the surfaces of implanted medical devices leads to increased patient morbidity and mortality and constitutes a global healthcare problem,” the researchers continued. “… inhibiting bacterial biofilm development on such devices to reduce the risk of chronic, persistent infections is a major unmet medical need.”
Significant effort has gone into reducing the likelihood of bacteria attaching to such devices by incorporating antibiotics and other antimicrobials into the plastics, but this approach does have drawbacks, including limited long-term efficacy and the development of antimicrobial resistance.
The ability of bacteria to attach to surfaces is sensitive to surface topography, the team further noted. However, while surface feature shape, height, and spacing are known to influence cell attachment, a general understanding of how topography achieves this is lacking. “There is therefore a need to determine mechanistically how topography modulates bacterial cellular responses,” they pointed out. “This knowledge gap hampers rational design of topographical biomaterials that could substantially improve the efficiency of discovery and the efficacy of their bacterial biofilm inhibitory properties.”
Through their newly reported study, the team screened more than 2000 designs made in different plastics including polyurethane, which is commonly used to make medical devices, to identify surface patterns that prevent biofilm formation.
“To discover design rules for topographical control of bacterial responses to surfaces (beyond simple geometries or isolated bioinspired topographical designs), we conducted an unbiased screen of combinatorially generated shapes using a high throughput microtopographical polymer chip, the TopoChip,” they explained. The investigators screened the TopoChip array against two important pathogens, Pseudomonas aeruginosa and Staphylococcus aureus. The screening results were then used to train machine learning (ML) models that mapped biological outcomes to surface properties. This then allowed the team to carry out iterative prediction and testing to help optimize the ‘hit’ topographies.
Williams noted, “Previous research has shown that introducing antibiotics to medical devices has flaws, such as driving the development of antibiotic resistance. Our study took this idea one step further as we wanted to find out if we could create a simple landscape on a catheter, made of the same material that bacteria didn’t like and couldn’t form biofilms on.”
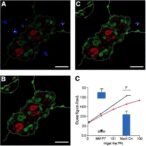
![Image shows cells becoming confined within the crevices between the features in the right-hand image after 2 hours and produce a lubricating substance which blocks subsequent biofilm formation. [University of Nottingham]](https://www.genengnews.com/wp-content/uploads/2025/06/low-res-8-300x300.jpeg)
The researchers found that most effective topographies significantly reduced attachment and subsequent biofilm formation in vitro by key pathogens, including Pseudomonas aeruginosa. Tests indicated that small crevices in the raised patterns trap the bacterial cells, tricking them into producing a lubricant that stops them sticking to the plastic surface. This in turn blocks biofilm formation and makes it easier for the host’s immune defenses cells to clear the infecting bacteria and so prevent infection.
Further experiments with implants in mouse models infected with P. aeruginosa also indicated that the most effective topographies retained their anti-attachment properties in vivo.
“Clearly, for biofilm inhibition, surface topographies associated with low pathogen attachment are required,” the investigators stated. “Our ML models have identified the most relevant topographical feature descriptors contributing to bacterial attachment.” The results, they pointed out, indicate that these micro-topographies have considerable potential for preventing biofilm formation in a clinical context.
Alexander, whose research focuses on the control of cells with polymer surfaces, said, “Using physically pattered surfaces has the advantage over coating approaches that it can be applied to existing device materials, reducing the barrier to commercial application. Our discovery could save the NHS a lot of money.” The authors added, “Texturizing the surface of clinically approved biomaterials would have the benefit of retaining their physical and mechanical properties while significantly reducing the need for expensive new materials discovery and commercial development.”
The researchers hope to expand this in current UK-RI funding to practical medical devices in collaboration with medical device companies.