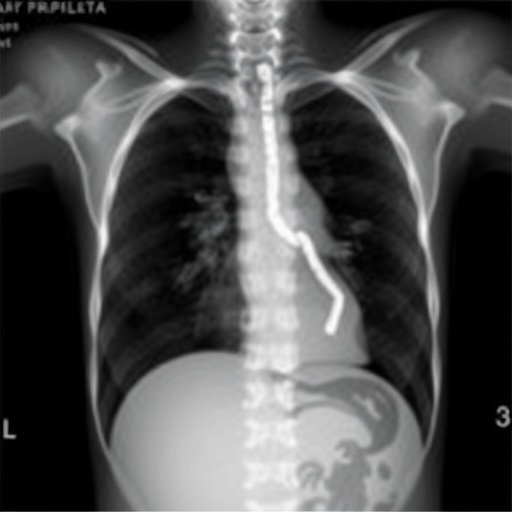
In a groundbreaking advancement that promises to reshape the landscape of neonatal respiratory care, researchers have unveiled novel insights into regional lung function abnormalities in congenital diaphragmatic hernia (CDH) using electrical impedance tomography (EIT). This cutting-edge imaging technique, known for its real-time, radiation-free monitoring capabilities, has provided unprecedented spatial and temporal detail on lung ventilation patterns in infants afflicted by this life-threatening congenital anomaly. The study, conducted by led by Douglas, Ferguson, and Tingay, and recently published in Pediatric Research (2025), shines a spotlight on the complex pulmonary pathophysiology underpinning CDH and charts a promising course toward more precise and individualized therapeutic interventions.
Congenital diaphragmatic hernia, a developmental defect characterized by incomplete formation of the diaphragm, allows abdominal organs to herniate into the thoracic cavity, compromising lung development and function. Infants born with CDH often face severe respiratory distress due to hypoplastic lungs and pulmonary hypertension. Despite advances in neonatal intensive care, mortality and morbidity remain high, primarily because of inadequate understanding and monitoring of lung function dynamics at the regional level within the compromised lungs. Traditional imaging modalities such as chest X-rays and computed tomography scans lack the resolution and safety profiles necessary for continuous bedside assessment, especially in fragile neonates.
This new investigative approach harnesses electrical impedance tomography — a noninvasive imaging technique that maps the distribution of electrical conductivity across the thorax to infer regional ventilation patterns. By analyzing impedance changes associated with inhaled air volumes, EIT offers a functional map of how different lung regions are ventilated over time without exposing the patient to ionizing radiation. The implications for managing infants with CDH are profound, as EIT could enable clinicians to track lung recruitment and detect ventilation heterogeneity dynamically, adjusting respiratory support strategies in real time.
.adsslot_fEeZqCWXwm{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_fEeZqCWXwm{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_fEeZqCWXwm{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The research team embarked on a detailed evaluation of regional lung function in a cohort of infants diagnosed with CDH, employing EIT immediately postnatal and during the course of intensive respiratory management. Their findings revealed that ventilation in affected lungs is not uniformly distributed; rather, there exist distinct zones of disproportionate aeration, which fluctuate with evolving clinical interventions. These heterogeneous ventilation patterns reflect the underlying structural abnormalities and mechanical stiffness caused by pulmonary hypoplasia, emphasizing the need for individualized ventilatory settings to minimize volutrauma and atelectasis.
Importantly, the study underscored the limitations of global respiratory parameters conventionally used in clinical practice, such as oxygenation indices and blood gas analysis, which inadequately represent the complex internal mechanics of a CDH-affected lung. Through EIT-derived regional indices, clinicians can visualize real-time ventilation distribution shifts, gaining insights into which lung segments are adequately aerated and which remain compromised. This level of detail allows for titration of positive end-expiratory pressure (PEEP) and other ventilation parameters with a tailored approach aimed at maximizing alveolar recruitment while minimizing ventilator-induced lung injury.
Moreover, this research marks a significant step toward early prognostication in CDH, as variations in regional lung function detected via EIT correlated with clinical outcomes such as duration of mechanical ventilation and survival rates. Early identification of lung regions prone to collapse or overdistension could guide therapeutic decision-making and prompt timely interventions, potentially improving long-term respiratory prognosis and reducing associated comorbidities. The ability to monitor lung function continuously at the bedside presents an invaluable tool for neonatologists attempting to navigate the precarious balance between adequate oxygenation and lung protection.
Technically, EIT in neonatal populations presents its own set of challenges, including the need for appropriately sized electrode arrays and the interpretation of data in a rapidly changing thoracic physiology. The authors innovated by adapting electrode placement specific to the small thoracic circumference of neonates with CDH and refined computational algorithms to enhance imaging resolution and artifact reduction. Their methodological advancements assure the robustness and reliability of EIT data, paving the way for its integration into routine clinical monitoring in neonatal intensive care units worldwide.
The clinical utility of this research extends beyond CDH, offering a model for the assessment of heterogeneous lung diseases in neonates. Conditions such as bronchopulmonary dysplasia and acute respiratory distress syndrome share the fundamental problem of uneven ventilation, which EIT can characterize dynamically. The noninvasive, radiation-free nature of EIT makes it especially attractive for repeated measurements necessary in fragile infants, allowing clinicians to track disease progression and response to therapy with unprecedented fidelity.
Beyond clinical implications, the study also contributes to refining the understanding of neonatal lung biomechanics. Data generated by EIT provide insight into how lung compliance and resistance vary across diseased segments, informing not only bedside strategies but also the development of predictive computational models of lung behavior. Such models could simulate various ventilatory strategies to optimize lung recruitment and reduce injury, ultimately leading to personalized respiratory management protocols informed by real-time functional imaging.
In addition to the direct impact on patient care, the research team highlights the potential integration of EIT data with other physiological monitoring modalities, such as near-infrared spectroscopy and capnography. This multimodal approach could offer a comprehensive view of pulmonary and systemic function, enhancing diagnostic precision and tailoring therapeutic interventions more effectively. The convergence of these technologies epitomizes the future of neonatal intensive care, where detailed physiological insights drive better outcomes.
As neonatal medicine strives toward precision and minimally invasive technologies, this study’s validation of EIT for regional lung function assessment in CDH patients heralds a paradigm shift. The ability to visualize and quantify lung ventilation heterogeneity in real time empowers clinicians to transcend the guesswork and anecdotal experience traditionally associated with ventilator management. This innovation aligns with broader trends toward functional phenotyping and personalized medicine in respiratory care, with important implications for survival and quality of life in this vulnerable population.
Nevertheless, the authors acknowledge that, while promising, EIT technology and its application in neonatal CDH require further refinement and multicentric validation to establish standardized clinical protocols. Future research will need to address long-term impacts of EIT-guided ventilation strategies on respiratory outcomes and neurodevelopment, as well as integrate EIT into decision-support algorithms harnessing artificial intelligence for automated interpretation and intervention recommendations.
This study’s ripple effect is poised to inspire a cascade of research exploring various congenital and acquired neonatal respiratory disorders with EIT, accelerating progress toward noninvasive, continuous, individualized respiratory monitoring. As neonatal intensive care embraces this technology, the ultimate beneficiaries will be the tiniest patients, whose fragile lungs stand to gain the clearest window yet into their function and healing potential.
In sum, the pioneering work by Douglas, Ferguson, and Tingay etches a powerful new chapter in neonatal respiratory care by demonstrating that electrical impedance tomography transcends mere imaging to become a vital functional tool in confronting one of the most formidable challenges—regional lung dysfunction in congenital diaphragmatic hernia. This innovation not only promises to improve survival and reduce morbidity but also exemplifies the transformative potential of integrating sophisticated physiological monitoring into clinical care for the most vulnerable patients.
Subject of Research: Regional lung function assessment in congenital diaphragmatic hernia using electrical impedance tomography.
Article Title: Regional lung function in congenital diaphragmatic hernia assessed using electrical impedance tomography.
Article References:
Douglas, E., Ferguson, K.N. & Tingay, D.G. Regional lung function in congenital diaphragmatic hernia assessed using electrical impedance tomography. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04185-9
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41390-025-04185-9
Tags: congenital diaphragmatic hernia assessmentelectrical impedance tomography in infantshypoplastic lungs in newbornsindividualized therapeutic interventions for CDHlung function monitoring techniquesneonatal intensive care advancementsneonatal respiratory care innovationspulmonary pathophysiology in CDHradiation-free imaging in pediatricsreal-time imaging for lung ventilationregional lung function abnormalities in CDHrespiratory distress in congenital anomalies