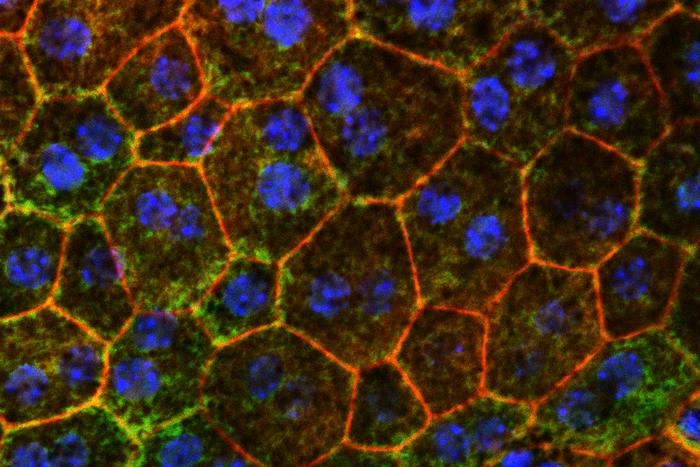
![Fixing problems with cholesterol metabolism might help slow or prevent a common cause of age-related vision loss, a new WashU Medicine study in mice has shown. Pictured are color-stained retinal epithelial cells from a mouse eye, the first cells to die as age-related macular degeneration progresses. [Tim Lee]](https://www.genengnews.com/wp-content/uploads/2025/06/low-res-12.jpeg)
Fixing problems with cholesterol metabolism might help slow or prevent a common cause of age-related vision loss, a new WashU Medicine study in mice has shown. Pictured are color-stained retinal epithelial cells from a mouse eye, the first cells to die as age-related macular degeneration progresses. [Tim Lee]
An international research team headed by scientists at Washington University School of Medicine in St. Louis has identified a possible way to slow or block progression of age-related macular degeneration (AMD), a leading cause of blindness in people over age 50 years. Their study implicated problems with cholesterol metabolism in this type of vision loss, perhaps helping to explain the links between macular degeneration and cardiovascular disease, which both worsen with age.
The new findings—identified using human plasma samples and mouse models of macular degeneration—suggest that increasing the amount apolipoprotein M (ApoM) in the blood may fix problems in cholesterol processing that lead to cellular damage in the eyes and other organs. The results indicate that various methods of dialing up ApoM could serve as new treatment strategies for age-related macular degeneration and perhaps some forms of heart failure triggered by similar dysfunctional cholesterol processing.
“Our study points to a possible way to address a major unmet clinical need,” said research co-lead Rajendra S. Apte, MD, PhD, the Paul A. Cibis Distinguished Professor of Ophthalmology and Visual Sciences at WashU Medicine. “Current therapies that reduce the chance of further vision loss are limited to only the most advanced stages of macular degeneration and do not reverse the disease. Our findings suggest that developing treatments that increase ApoM levels could treat or even prevent the disease and therefore preserve people’s vision as they age.”
Co-senior author Apte and colleagues reported on their study in Nature Communications, in a paper titled “Apolipoprotein M attenuates age-related macular degeneration phenotypes via sphingosine-1-phosphate signaling and lysosomal lipid catabolism.”
Age-related macular degeneration (AMD) is the leading cause of blindness among Americans over 50, the authors noted. In macular degeneration, doctors can see cholesterol-rich deposits under the retina during an eye exam, according to Apte. “In its early and intermediate stages, AMD is marked by the buildup of lipoprotein-rich deposits—either under the neurosensory retina (sub-retinal drusenoid deposits, or SDD) or beneath the retinal pigment epithelium (RPE), known as drusen,” the authors further explained. In these early stages, vision might still be normal, but the deposits increase inflammation and other damaging processes that lead to the gradual loss of central vision.
In the most common, “dry” macular degeneration form, the cells in the central part of the retina can be damaged, causing a type of neurodegeneration called geographic atrophy (GA), which is similar to what happens in the brain in conditions such as Alzheimer’s disease. The other form of AMD is known as “wet” AMD, in which abnormal blood vessel growth (choroidal neovascularization or CNV) damages vision. Although it represents only about 20% of advanced cases, wet AMD accounts for roughly 80% of AMD-related vision loss, the authors continued.
Geographic atrophy and wet macular degeneration are advanced forms of the disease that are accompanied by vision loss. “Current treatments are limited to the advanced stages of the disease,” the team further noted. However, the disease process itself is not reversible at that stage.
“Recent studies, including work from our laboratory, have highlighted the pivotal role of cholesterol and lipid accumulation in the initiation and progression of AMD,” the authors further reported. In recent years, evidence has also emerged that ApoM can serve as a protective molecule with known anti-inflammatory effects and roles in maintaining healthy cholesterol metabolism. With that in mind, Apte and co-senior author Ali Javaheri, MD, PhD, an assistant professor of medicine, were interested assessing whether reduced ApoM levels, which fall with age, could be involved in the dysfunctional cholesterol metabolism that is at the root of multiple diseases of aging, including macular degeneration and heart disease. “Based on its involvement in aging-related disease and cholesterol metabolism, we hypothesized that the lipocalin apolipoprotein M (ApoM) may play an important role in AMD,” they stated. ApoM binds directly to sphingosine-1-phosphate (S1P), a bioactive sphingosine that activates S1P receptors found on various cell types, including retinal pigment epithelial (RPE) cells.
The investigators’ studies showed that patients with macular degeneration have reduced levels of ApoM circulating in the blood compared with healthy controls. And past work by Javaheri, a WashU Medicine cardiologist, showed that patients with various forms of heart failure also had reduced levels of ApoM in the blood.
This study revealed that ApoM is a key component in the “good cholesterol” pathways that mop up excess cholesterol—the bad kind that tends to drive inflammation—and clear it from the body through the liver.
Apte and Javaheri’s research suggests that when ApoM is low, cells in the retina and heart muscle can’t correctly metabolize cholesterol deposits and have a hard time getting rid of these accumulating lipids. Build up of these lipids leads to inflammation and cellular damage.
To see if they could reverse the harmful effects of low ApoM, the researchers increased ApoM levels in mouse models of macular degeneration, using genetic modification or plasma transfer from other mice. The mice showed evidence of improved retinal health, improved function of light-sensing cells in the retina and reduced accumulation of cholesterol deposits. “We demonstrated that several pathogenic features observed in murine models of dry AMD, such as RPE and rod photoreceptor dysfunction and RPE lipid accumulation, are improved by ApoM,” they stated. “We also demonstrated that the salutary effects of ApoM are contingent on S1P binding.”
The researchers further described evidence that ApoM triggers a signaling pathway that breaks down the cholesterol in cellular compartments called lysosomes, which are known for playing important roles in disposing of cellular waste. They in addition also found that ApoM must be bound to S1P to get the beneficial effects of ApoM treatment in the mice.
“This study as a whole supports the notion that ApoM-S1P signaling upregulates lysosomal acid lipase activity, a mechanism that warrants further investigation,” they stated.
The findings might have implications for future interventions that raise ApoM in patients with heart failure. Javeheri said, “One of the exciting things about this collaboration is realizing the links between retinal pigment epithelial cells and heart muscle cell, which are both vulnerable to low ApoM. It is possible that the interaction between ApoM and S1P is regulating cholesterol metabolism in both cell types. We look forward to exploring strategies to increase ApoM in ways that could help the eye and the heart maintain healthy cholesterol metabolism over time and stave off two major diseases of aging.” In their paper the team concluded, “Our study, specifically focused on addressing the fundamental mechanisms of RPE lipotoxicity, highlights a novel therapeutic use of ApoM-S1P to prevent RPE lipotoxicity via enhanced elimination of lipids and deserves further exploration.”
Apte and Javaheri are working with Mobius Scientific, a WashU startup company that is working to harness this knowledge of the role of ApoM in macular degeneration to develop new approaches to treating or preventing the disease. Apte and Javaheri worked with WashU’s Office of Technology Management (OTM) to launch Mobius Scientific in 2022.