In a groundbreaking advance at the intersection of quantum physics and nanophotonics, researchers from the Bionanophotonic Systems Laboratory at EPFL’s School of Engineering have unveiled a revolutionary biosensor that operates without the need for an external light source. This new device harnesses a quantum phenomenon known as inelastic electron tunneling to generate and detect light on a nanoscale chip, offering unparalleled sensitivity for biomolecule detection. The technology not only challenges the traditional reliance on bulky and expensive optical equipment but could also pave the way for portable, real-time diagnostic tools in medicine and environmental monitoring.
Optical biosensors have long been pivotal in scientific and medical fields due to their ability to detect molecules using light waves. These sensors function by probing biological samples, offering insights critical for personalized medicine, early disease diagnosis, and pollution monitoring. However, a persistent challenge has been to confine light waves to the nanometer scale—dimensions comparable to individual proteins or amino acids—to improve detection sensitivity. Conventional methods employ intricate nanophotonic structures that “squeeze” light at the surface of a chip, but these systems typically necessitate external lasers or light sources, resulting in complex and costly instrumentation unsuitable for rapid or point-of-care applications.
Turning to quantum mechanics provided the breakthrough. The team’s innovation rests on exploiting inelastic electron tunneling, a phenomenon where electrons, considered as waves rather than mere particles, have a finite probability of traversing an ultra-thin insulating barrier, simultaneously emitting photons—packets of light—in the process. Engineering a nanostructure that both composes part of the tunneling barrier and enhances photon emission probability was key to transforming this subtle quantum effect into a practical light source embedded directly within the sensor.
.adsslot_JPj5A1RWuz{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_JPj5A1RWuz{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_JPj5A1RWuz{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
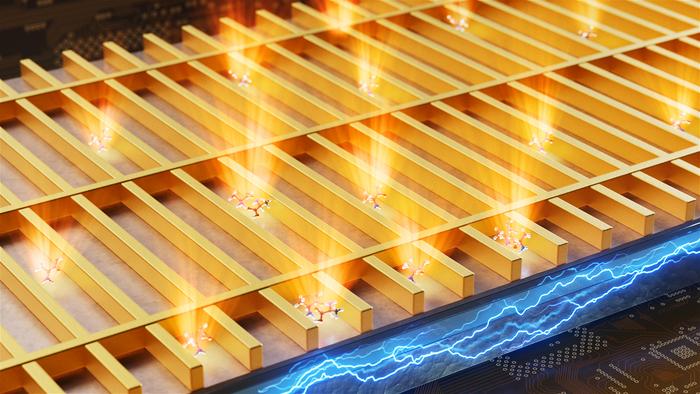
At the heart of the device’s architecture lies a meticulously designed nanoscale assembly comprising an aluminum oxide insulating layer and an ultrathin gold film. When electrons are driven through the aluminum oxide by applying a voltage, they occasionally tunnel across this barrier into the gold. This tunneling event transfers energy to collective electron oscillations within the gold—plasmons—which subsequently relax by emitting photons. Notably, the intensity and spectral characteristics of this photon emission shift in response to the interaction with biomolecules on the sensor’s surface, effectively translating biological information into an optical signal without the need for fluorescent labels or external lasers.
The sensor’s core innovation is its gold metasurface, fashioned as an arrayed mesh of nanoscale gold wires acting as optical nanoantennas. This metasurface serves dual purposes: it forms part of the quantum tunneling junction and simultaneously governs the spatial and spectral distribution of the emitted light. By concentrating light into nanometric volumes exactly where biomolecules can interact, these nanoantennas significantly amplify detection sensitivity and specificity, enabling the device to discern molecular phenomena at previously unreachable scales.
Despite the inherently low-probability nature of inelastic electron tunneling, the researchers ingeniously countered this by scaling the process over a macroscopic area. By integrating the quantum tunneling mechanism uniformly across a sizeable surface, the biosensor accumulates sufficient photon emission to generate meaningful signals, overcoming a fundamental limitation. This approach contrasts sharply with traditional single-point detection methods, exemplifying a promising blueprint for future quantum-enabled sensing platforms.
Performance evaluations of the biosensor demonstrated its ability to detect amino acids and polymers at concentrations in the picogram range—equivalent to one trillionth of a gram. Such sensitivity rivals or even exceeds that of current cutting-edge biosensors, underscoring the system’s potential for real-world applications. Furthermore, the detection is label-free and occurs in real time, a significant advantage for clinical diagnostics and environmental monitoring where speed and ease of use are paramount.
Fabrication leveraged EPFL’s state-of-the-art Center of MicroNanoTechnology facilities, ensuring that the sensor is not only highly functional but also scalable, compatible with established manufacturing techniques, and compact. The active sensing area encompasses less than a square millimeter, heralding the feasibility of integrating these biosensors into handheld devices for decentralized and rapid testing scenarios. Such portability could be transformative for healthcare delivery in resource-limited settings and for on-site detection of environmental pollutants.
This technology represents a synthesis of multiple advanced scientific concepts. The interplay between quantum electron behavior, plasmonic resonances of nanostructured metals, and precise nanofabrication has yielded a new class of biosensors capable of merging light generation and detection into a single integrated chip. The seamless coalescence of these functions eliminates bulky optical setups and lowers barriers to widespread deployment.
Collaborations with leading institutions worldwide, including ETH Zurich, ICFO in Spain, and Yonsei University in Korea, attest to the global significance and multidisciplinary nature of this breakthrough. The findings were recently published in the prestigious journal Nature Photonics, an acknowledgment of both the scientific rigor and the high potential impact of the work.
Looking ahead, the quantum plasmonic biosensor platform opens numerous avenues for innovation. Beyond medical diagnostics and environmental sensing, the fundamental scientific insights could influence a broader array of fields such as quantum computing, nano-optics, and materials science. The concept of harnessing quantum tunneling for integrated light generation signals a paradigm shift in photonic device engineering.
In summary, this self-illuminating plasmonic biosensor stands as a pioneering example of how quantum mechanics can transcend theoretical curiosities, evolving into practical, scalable technologies with societal relevance. By embedding quantum light sources directly into chip-scale devices, the researchers have created a new frontier in biosensing technology—one that promises unprecedented sensitivity, compactness, and versatility across numerous domains.
Subject of Research: Quantum plasmonic biosensors utilizing inelastic electron tunneling for sensitive biomolecule detection
Article Title: Plasmonic biosensor enabled by resonant quantum tunnelling
News Publication Date: 26-Jun-2025
Web References: https://doi.org/10.1038/s41566-025-01708-y
References: Masharin et al., Nature Photonics, 2025
Image Credits: 2025 Ella Maru Studio/BIOS EPFL CC BY SA 4.0
Tags: advancements in optical biosensorsbiomolecule detection technologychallenges in nanoscale light confinementcost-effective biosensing solutionsEPFL research breakthroughsinelastic electron tunneling applicationsnanophotonics in medicinepersonalized medicine innovationsportable diagnostic toolsquantum physics in biosensingreal-time environmental monitoringself-illuminating biosensor