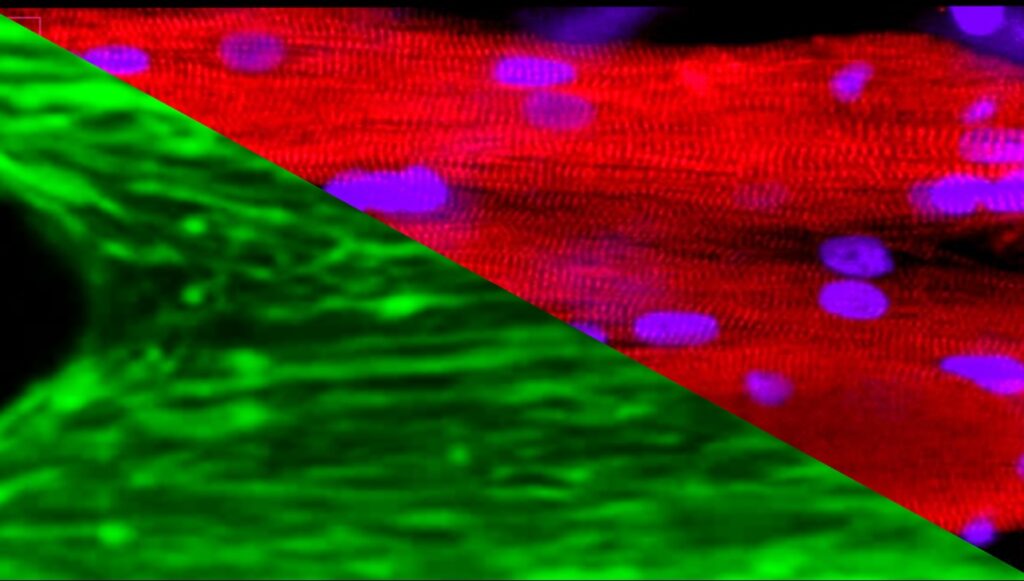
3D Donor-Derived Skeletal Muscle Tissue in custom microfluidic device compatible with spaceflight hardware [Maddalena Parafati (Malany`s Lab)]
Space exploration activity has been increasing. In turn, researchers are more interested than ever in uncovering more about how space travel affects human health and the progression of disease. Although it is known that microgravity accelerates skeletal muscle degeneration, the effects of space on muscle cell function remain unclear.
Space flight with the associated absence of gravity and limited strain on muscles causes muscle weakness within a short period of time, providing a time-lapse view of age-related atrophy-associated changes in the muscle. To understand the changes of muscle in microgravity, researchers from the University of Florida engineered skeletal muscle microtissues from donor biopsies and launched them to the International Space Station (ISS) aboard SpaceX CRS-25.
The findings are published in Stem Cell Reports in the paper, “Microgravity Accelerates Skeletal Muscle Degeneration: Functional and Transcriptomic Insights from an ISS Muscle Lab-on-Chip Model.”
The study shows that muscle decline can be modeled within a relatively short period in space and paves the way for follow-up studies on causes and potential treatments for diseases like sarcopenia from aging.
Using a muscle lab-on-chip model onboard the ISS, the researchers examined 3D-bioengineered myobundles derived from young and older adult donors under microgravity. The microtissues were cultured in an automated mini lab, which enabled electrical stimulation to simulate exercise. More specifically, electrical stimulation applied intermittently to the myobundles “revealed reduced contraction magnitude in microgravity and decreased protein levels of myosin heavy chain 7, a main isoform in slow-twitch muscle fibers,” the authors wrote.
“Using electrical pulses to trigger real-time muscle contractions in space, we can simulate exercise and observe how it helps protect against rapid muscle weakening in microgravity,” said Siobhan Malany, PhD, associate professor at the University of Florida College of Pharmacy. “This technology advancement offers insight into how we might preserve muscle health during long-duration space missions and ultimately, how to combat age-related muscle loss here on Earth.”
The team used transcriptomic profiling to reveal active myogenesis across ground and spaceflight samples. In turn, they found that younger electrically stimulated myobundles displayed enhanced mitochondrial-related gene expression in microgravity. In contrast, older (and non-electrically stimulated) myobundles were less responsive.
The team then identified 86 muscle-specific age-associated genes altered in microgravity by performing a comparative analysis between young and older derived myobundles. The genes were linked to inflammation, mitochondrial dysfunction, and cellular stress.
These findings not only reveal a unique age-related molecular response in microgravity and underscore electrical stimulation as a potential countermeasure but they also advance the understanding of muscle aging and degeneration in microgravity, guiding future therapeutic strategies.