A groundbreaking study recently published in the distinguished journal Oncotarget unveils critical insights into the complex molecular architecture of HER2-mutated non-small cell lung cancer (NSCLC) within the underrepresented population of Northeastern Brazil. This research highlights the profound genetic heterogeneity and clinical challenges posed by HER2 mutations in NSCLC, an area that has historically seen limited exploration outside high-income countries. The work, spearheaded by a collaborative team from the Federal University of Ceará, Argos Pathology Laboratory, and Messejana Heart and Lung Hospital, sheds new light on the diagnostic intricacies and therapeutic potential that could inform the future management of lung cancer globally.
HER2, also known as ERBB2, is a well-established oncogene implicated in a variety of malignancies, most notably breast and gastric cancers. However, its role in lung cancer—particularly NSCLC—has emerged more recently, revealing a niche yet vital subset of patients whose tumors harbor activating mutations or amplifications of this gene. The current investigation focuses on a cohort of 13 patients from Northeastern Brazil with identified HER2 mutations in NSCLC tumors, drawing attention not only to the presence of these mutations but also to their extensive genomic interplay with other oncogenic drivers. This nuanced molecular profiling propels the study beyond mere identification, providing a window into the intricate mutational landscapes that drive tumor behavior in this demographic.
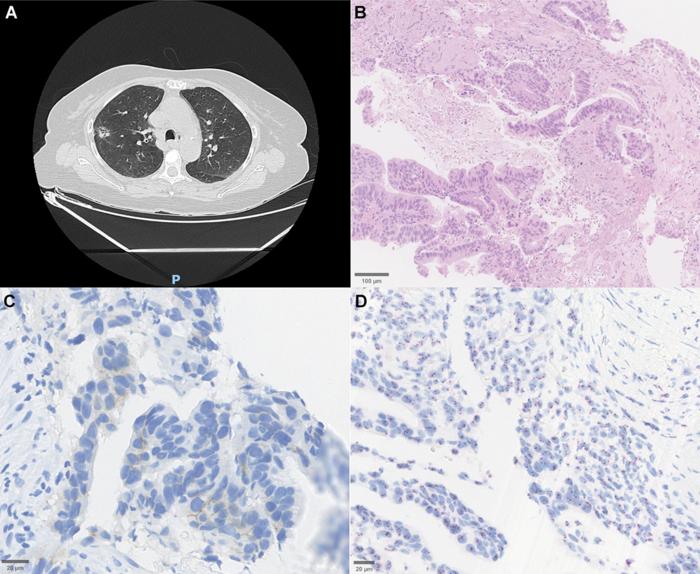
The cohort ranged widely in age from 34 to 82 years, with a slight female predominance and nearly half classified as non-smokers—a noteworthy epidemiological feature given the traditionally strong association of lung cancer with tobacco exposure. Morphologically, the tumors exhibited classic adenocarcinoma patterns, with histopathological examination detailing mixed acinar and papillary architectures. Notably, immunohistochemical analysis revealed low-level HER2 protein expression despite DNA-level mutations, a finding underscored by dual in situ hybridization techniques confirming the absence of gene amplification. These subtleties emphasize the complexity inherent in relying solely on protein expression or amplification status to define HER2 involvement in NSCLC.
.adsslot_V1g2zenwxc{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_V1g2zenwxc{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_V1g2zenwxc{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Genomic interrogation uncovered a rich tapestry of co-occurring mutations. Alterations in TP53, KRAS, and STK11 genes frequently accompanied HER2 mutations, underscoring the multifaceted oncogenic networks that coalesce within these tumors. Among the HER2 mutations identified, exon 20 insertions predominated, conforming to a hotspot region known to induce constitutive activation of the HER2 tyrosine kinase domain. This mechanistic detail furthers our understanding of how these mutations contribute to malignant transformation and progression in NSCLC, offering tangible targets for pharmacologic intervention.
From a therapeutic standpoint, the study paints a sobering picture of current clinical realities. Despite the availability of promising HER2-targeted agents, notably trastuzumab deruxtecan (T-DXd)—the first FDA-approved drug to demonstrate robust efficacy in HER2-mutant NSCLC—the majority of patients in this cohort did not receive targeted therapy. Instead, conventional modalities such as surgery, chemotherapy, and immunotherapy were predominantly employed, reflecting both access barriers and the necessity for heightened awareness and infrastructure to implement molecular testing and precision medicine strategies in low-resource settings.
The disparity in treatment outcomes was stark. Survival times ranged widely, with some individuals achieving protracted remission extending several years post-diagnosis, while others succumbed rapidly, within months. This heterogeneity not only mirrors the underlying biological complexity revealed by the molecular data but also highlights the critical importance of early, accurate diagnosis paired with the optimal therapeutic approach. The authors rightly advocate for integrating comprehensive molecular profiling into routine diagnostic algorithms, advocating a tiered strategy that begins with baseline screening for actionable mutations followed by more sophisticated genomic analyses when warranted.
Importantly, this study addresses a significant gap in cancer research by spotlighting a population that has been historically marginalized in genomic and clinical oncology research. Latin America, and particularly regions like Northeastern Brazil, are underrepresented in large-scale cancer genomics initiatives. This lack of representation constrains global understanding of disease biology and impedes equitable drug development and clinical guideline formulation. By elucidating the unique molecular features and clinical patterns of NSCLC with HER2 mutations in this geographic and ethnic context, the research elevates the imperative for inclusive, region-specific cancer research endeavors.
The study’s findings also provoke reflection on the molecular diagnostic tools currently available and their performance in diverse populations. The relatively low HER2 protein expression despite the presence of activating mutations invites reconsideration of reliance on immunohistochemistry (IHC) alone for patient selection in clinical trials and therapeutic decision-making. Dual in situ hybridization assays and next-generation sequencing (NGS) emerge as indispensable adjuncts to uncover the full spectrum of HER2 alterations and accompanying mutations, thereby refining patient stratification and enhancing personalized medicine.
Moreover, the research team’s proposal of a tiered diagnostic framework tailored to resource availability and clinical need represents a pragmatic model with potential applicability far beyond the study’s geographic confines. Starting with cost-effective screening methods and escalating to detailed genomic profiling as indicated, such an approach could democratize access to precision oncology and improve clinical outcomes through appropriate therapeutic sequencing.
This research further underscores the transformative potential of novel HER2-directed therapies like trastuzumab deruxtecan, which have demonstrated significant response rates and progression-free survival benefits in HER2-mutant NSCLC. However, the limited use of these agents within the studied cohort delineates pressing access issues. Addressing these gaps necessitates concerted efforts to build infrastructure, training, and healthcare policies that promote molecular testing and equitable distribution of targeted treatments, especially in low- and middle-income countries.
Future directions prompted by this work include expanding cohort sizes to validate findings and unravel prognostic implications of different HER2 mutation subtypes. Additionally, longitudinal studies integrating clinical outcomes with molecular profiles will be crucial to ascertain predictive biomarkers of treatment response and resistance mechanisms. Ultimately, these endeavors will inform guidelines that are both globally relevant and locally adaptable.
In addition to clinical and molecular insights, the study carries socio-ethical ramifications, emphasizing the need for global oncology communities to prioritize underrepresented populations to achieve health equity. The research thus serves as a clarion call to dismantle barriers that have historically limited access to cutting-edge cancer diagnostics and therapeutics worldwide.
In conclusion, this seminal investigation charts critical molecular and clinical terrain by decoding the complex landscape of HER2-mutated NSCLC in Northeastern Brazil. By highlighting the intricate genomic interplay, diagnostic hurdles, treatment disparities, and the pressing need for expanded molecular profiling and targeted therapy access, it propels the field toward more inclusive, precise, and effective lung cancer management strategies. The study not only enriches scientific understanding but also reinforces the broader imperative of global health equity in oncology research and care.
Article Title: Molecular landscape of HER2-mutated non-small cell lung cancer in Northeastern Brazil: Clinical, histopathological, and genomic insights
News Publication Date: 17-Jun-2025
Web References:
Oncotarget Volume 16: https://www.oncotarget.com/archive/v16/
DOI: 10.18632/oncotarget.28737
Image Credits: Copyright: © 2025 Nogueira et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Keywords: cancer, HER2 mutation, NSCLC, lung cancer, targeted therapy, genomic profiling
Tags: collaborative cancer research in Brazildiagnostic challenges in HER2 NSCLCgenetic heterogeneity in NSCLCgenomic profiling of lung tumorsHER2 mutations in lung cancermolecular architecture of lung cancernon-small cell lung cancer disparitiesNortheastern Brazil lung cancer researchoncogene ERBB2 in malignanciestherapeutic implications of HER2 mutationstreatment disparities in cancer careunderrepresented populations in cancer studies