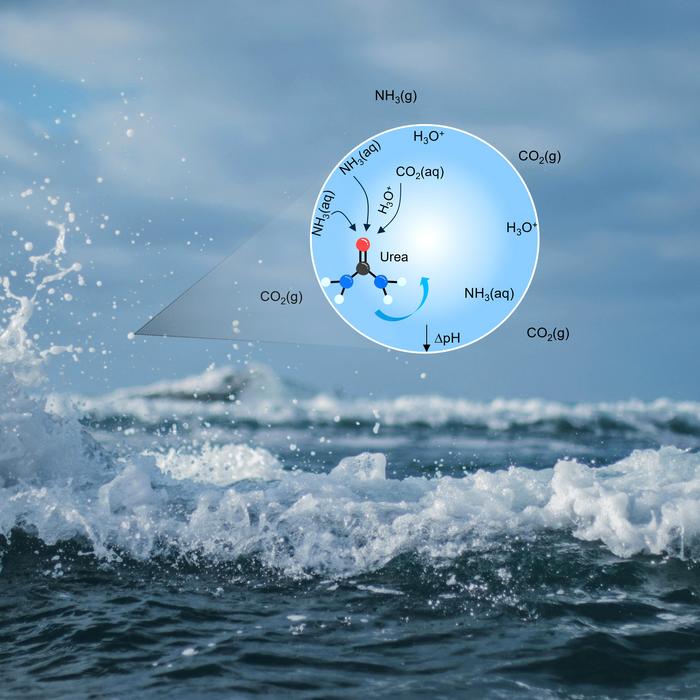
In a groundbreaking study that could reshape our understanding of prebiotic chemistry and the origins of life on Earth, researchers from ETH Zurich have unveiled a previously unknown pathway by which urea—the fundamental organic compound critical to both industrial applications and biological systems—can form under ambient environmental conditions. This discovery challenges longstanding assumptions by demonstrating that urea can spontaneously emerge through a reaction between carbon dioxide (CO₂) and ammonia (NH₃) within the microscopic water droplets suspended in the atmosphere, such as sea spray and mist. The team’s findings, recently published in the prestigious journal Science, reveal that the unique chemical environment at the air-water interface dramatically alters reaction kinetics, enabling a process that previously required high temperatures, pressures, or catalysts.
Urea, known chemically as CO(NH₂)₂, occupies a central place in the world of chemistry. Industrially, it serves as a vital fertilizer, a precursor to synthetic resins and explosives, and as a fuel additive that reduces harmful nitrogen oxides in vehicle emissions. Its biological significance is equally profound: urea is a key nitrogenous waste product metabolized in living organisms, and it has been proposed as a crucial building block in the emergence of complex biomolecules like RNA and DNA. Despite its importance, the precise prebiotic pathways through which urea itself could have originated on the early Earth’s harsh and largely enigmatic environment have remained unresolved—until now.
The research spearheaded by Professor Ruth Signorell, an expert in physical chemistry, probes the dynamic interface between aqueous droplets and gas-phase molecules. Typically, synthesizing urea industrially requires catalytic conditions with elevated temperature and pressure to drive the chemical reaction between ammonia and carbon dioxide. Biological systems circumvent these harsh conditions through enzymatic catalysts. However, the ETH Zurich team concentrated on natural microenvironments created by droplets that are omnipresent in Earth’s atmosphere. These droplets, ranging in size from tens of nanometers to micrometers, possess an interface that functions like a highly specialized reactor where gases and liquids meet and interact in ways that differ significantly from bulk liquid chemistry.
.adsslot_gGTpZCxJty{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_gGTpZCxJty{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_gGTpZCxJty{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Their experiments revealed that when CO₂ and NH₃ are present at the boundary layer of these droplets, spontaneous formation of urea occurs with no added energy input. This phenomenon is attributed to the unique physicochemical gradients present at the droplet surface—particularly pH variations—that induce localized acidic microenvironments. The surface, thus, facilitates unconventional pathways that are thermodynamically unfavorable or kinetically hindered in homogeneous aqueous phases. These aqueous aerosols act as chemical microreactors, providing an amplified surface-to-volume ratio that accelerates concentration gradients and reaction rates beyond what would occur in bulk solution.
The implications of such findings stretch far beyond atmospheric chemistry. From an astrobiological perspective, this spontaneous urea synthesis provides a plausible prebiotic route for the accumulation of nitrogenous organic molecules essential for the origin of life. The early Earth’s atmosphere, understood to be rich in CO₂ and trace ammonia, combined with abundant aqueous aerosols from oceans and rivers, would have created the perfect milieu for such surface chemistry. This shifts the paradigm of prebiotic molecular evolution toward recognizing the critical role of interfaces and microenvironments in driving complex chemical syntheses relevant to life’s beginnings.
Complementing the experimental work, theoretical calculations conducted by collaborators at Auburn University substantiated the observed reaction mechanism. These computations validated that urea formation on droplet interfaces could occur spontaneously without external energy inputs, reinforcing the plausibility of these reactions under early Earth conditions. Such computational support bridges molecular-level understanding with lab-scale phenomena, offering robust evidence of the reaction pathway and its energetics.
Furthermore, this study adds a new dimension to the understanding of atmospheric aerosol chemistry, suggesting that natural atmospheric particles could not only influence climate and weather but also serve as chemical reactors with a significant role in global biogeochemical cycles. If aerosols naturally facilitate reactions like urea synthesis, they might contribute directly to the inventory of bioavailable nitrogen compounds, thereby influencing ecological and evolutionary dynamics in ways previously unappreciated.
The technological ramifications of this discovery are equally compelling. The ability to synthesize urea at ambient temperatures and pressures without catalysts heralds new possibilities for sustainable chemical manufacturing. Industrial production of urea currently consumes substantial energy and requires high-pressure reactors. By harnessing the principles unveiled in this study, it may be possible to develop greener, more energy-efficient methods for producing urea and its derivatives, contributing to climate-friendly chemical processes.
The importance of these findings is underscored by the role urea plays across diverse contexts—from agriculture to medicine to materials science. Understanding how urea can form naturally under mild conditions sheds light on a chemical cornerstone that bridges the inorganic and organic worlds. It also opens new research directions in the study of the physicochemical properties of interfaces and their contribution to chemical evolution.
Overall, the ETH Zurich team’s research advances a compelling narrative that redirects focus from energy-intensive bulk phase reactions to the overlooked yet vibrant chemistry at interfaces—microscopic stages where molecules collide, concentrate, and react in ways that can initiate the building blocks of life itself. As Professor Signorell articulates, this work exemplifies how seemingly mundane boundaries in nature transform into dynamic zones of chemical creativity, potentially uniting the origins of biological molecules under a common, interface-mediated mechanism.
In conclusion, the spontaneous formation of urea at the air-water interface under ambient conditions not only illuminates possible pathways for prebiotic chemistry but also challenges established industrial and environmental paradigms. This convergence of physical chemistry, atmospheric science, and prebiotic research signifies a pivotal step forward in deciphering the complex interplay between the environment and the emergence of life’s building blocks. The findings invite further exploration into the vast realm of interface chemistry, extending its implications from the primordial Earth to modern technological applications.
Subject of Research: Not applicable
Article Title: Spontaneous formation of urea from carbon dioxide and ammonia in aqueous droplets
News Publication Date: 26-Jun-2025
Web References:
https://ethz.ch/en/news-and-events/eth-news/news/2023/06/how-urea-may-have-been-the-gateway-to-life.html
https://www.science.org/doi/10.1126/science.adv2362
References:
Signorell, R., Mohajer Azizbaig, M., Basuri, P., Miliordos, E., Evdokimov, A., et al. “Spontaneous formation of urea from carbon dioxide and ammonia in aqueous droplets.” Science, 2025. DOI: 10.1126/science.adv2362
Image Credits: Luis Quintero / ETH Zürich
Keywords: Urea synthesis, prebiotic chemistry, atmospheric aerosols, air-water interface, spontaneous reactions, carbon dioxide, ammonia, Early Earth, origin of life, physical chemistry, aqueous droplets, sustainable chemical production
Tags: air-water interface chemistryatmospheric chemical processescarbon dioxide ammonia reactionenvironmental chemistry breakthroughsETH Zurich research findingsnitrogenous waste productsorigins of life researchprebiotic chemistry discoveriesRNA DNA building blockssignificance of urea in industryspontaneous urea formationurea in biological systems