In the realm of oncology, precision is not just a goal but a necessity. Radiation therapy, a cornerstone treatment for many cancer patients, hinges on the ability to accurately delineate tumor boundaries to deliver lethal doses of radiation to cancer cells while preserving surrounding healthy tissue. Despite advances in medical imaging, the process of tumor segmentation—the task of outlining the tumor’s exact location and volume—remains predominantly manual, labor-intensive, and susceptible to inter-observer variability. This traditional approach, though clinically indispensable, is fraught with challenges, including delays in treatment planning and potential oversights of critical tumor regions.
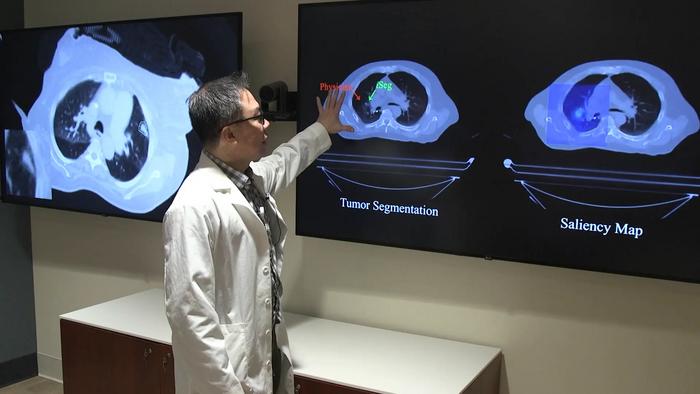
A team of researchers from Northwestern Medicine has introduced a groundbreaking artificial intelligence (AI) tool named iSeg that promises to revolutionize tumor segmentation in radiation oncology. Unlike previous AI models that analyzed static scans, iSeg employs advanced three-dimensional deep learning techniques to automatically segment lung tumors dynamically as they move with each breath. This innovation is particularly significant because lung tumors are subject to continuous motion due to respiration, complicating precise targeting during radiation therapy. By factoring in tumor movement, iSeg offers a more physiologically accurate representation, potentially improving treatment accuracy and patient outcomes.
The development of iSeg involved training on an extensive dataset comprising computed tomography (CT) scans from hundreds of lung cancer patients across nine clinical centers, including Northwestern Medicine and Cleveland Clinic health systems. Each scan was paired with tumor outlines meticulously drawn by expert oncologists, allowing the AI to learn from diverse clinical practices and imaging variations. This multi-institutional dataset surpasses the scale and heterogeneity of many previous studies, enhancing the tool’s robustness and generalizability across different hospital settings and scanner types.
.adsslot_bCposMtkfI{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_bCposMtkfI{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_bCposMtkfI{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
After rigorous training, iSeg was evaluated on previously unseen patient scans, comparing its tumor segmentations against those produced by radiation oncologists. The results were striking: iSeg’s contours consistently matched expert delineations with high accuracy and, crucially, identified tumor regions that some physicians had overlooked. These missed areas, if untreated, correlate with poorer prognoses, underscoring the clinical significance of the AI’s enhanced detection capability and suggesting that automated segmentation could become an essential adjunct in radiotherapy planning.
Accurate tumor targeting is foundational to the efficacy and safety of radiation treatment. Minor inaccuracies can result in insufficient tumor control or unintended damage to healthy tissues, compromising patient outcomes. Dr. Mohamed Abazeed, chair and professor of radiation oncology at Northwestern University Feinberg School of Medicine, emphasizes that tools like iSeg could transform the landscape of cancer treatment by boosting precision. By standardizing tumor contouring, this technology not only streamlines workflow but also ensures fairness and consistency across different clinical environments, which can be especially impactful in institutions lacking specialized expertise.
The real-time application of iSeg in clinical settings is already underway, as the research team works to integrate the AI tool into routine practice. This includes deploying iSeg in live comparisons alongside physicians’ segmentations and incorporating user feedback mechanisms to refine its performance further. Additionally, efforts are focused on adapting the platform beyond lung cancer to encompass other malignancies such as liver, brain, and prostate tumors, as well as broadening compatibility with various imaging modalities like magnetic resonance imaging (MRI) and positron emission tomography (PET) scans.
A distinguishing feature of iSeg is its capacity to model tumor motion caused by respiration, a persistent obstacle in thoracic radiotherapy. Traditional approaches often rely on static images or averaged tumor positions, risking radiation misses due to the dynamic nature of lung tumors. By contrast, iSeg’s motion-resolved segmentation accounts for this complexity, offering a four-dimensional perspective that aligns radiation delivery precisely with tumor movement. This advancement could lead to tailored radiation doses that maximize tumor eradication while minimizing collateral damage, thus enhancing therapeutic efficacy.
The AI model leverages convolutional neural networks optimized for volumetric data, enabling it to interpret spatial and temporal information embedded in CT imaging sequences. Through layers designed to capture intricate tumor boundaries and motion patterns, iSeg synthesizes a detailed tumor map that evolves through respiratory cycles. Such sophisticated deep learning architectures require enormous computational resources and careful tuning but deliver superior accuracy and adaptability compared to conventional manual contouring or simpler algorithmic segmentation methods.
In addition to technical sophistication, iSeg also embodies a move toward democratizing advanced cancer care. As automated tools become more reliable, they hold promise for extending high-precision radiation therapy capabilities to community clinics and regions where access to subspecialty oncologists is limited. This could reduce disparities in cancer treatment outcomes and ensure that patients receive uniformly excellent care regardless of geographical or institutional differences.
The significance of iSeg also resonates in its potential to hasten clinical workflows. Manual segmentation is time-consuming, often delaying the initiation of radiation therapy. Automated segmentation can drastically shorten this interval, facilitating quicker treatment planning and ultimately improving survival chances, especially for aggressive or rapidly progressing tumors. When deployed in clinical practice, iSeg’s efficiency is expected to complement rather than replace oncologists, allowing them to focus more on personalized patient care and decision-making.
Looking ahead, the research team envisions a future where AI-driven tools like iSeg become integral to oncology practice, continuously learning from accumulating data and adapting to emerging clinical challenges. Integration with multidisciplinary treatment planning systems and electronic health records could create a seamless environment for precision medicine, combining imaging, genomics, and patient-specific factors to tailor therapies with unprecedented specificity.
In summary, iSeg represents a leap forward in the application of AI for cancer treatment, embodying the convergence of medical expertise, imaging technology, and machine learning innovation. By automating the complex task of motion-resolved tumor segmentation, this tool holds the promise of enhancing radiation therapy’s precision, improving patient outcomes, and paving the way for smarter, faster, and more equitable cancer care worldwide.
Subject of Research: AI-driven motion-resolved tumor segmentation in radiation therapy
Article Title: Deep learning for automated, motion-resolved tumor segmentation in radiotherapy
News Publication Date: 30-Jun-2025
Web References:
DOI: 10.1038/s41698-025-00970-1
Image Credits: Northwestern University
Keywords: Radiation therapy, Cancer treatments, Artificial intelligence
Tags: advancements in medical imaging for oncologyAI in radiation oncologyAI tools in healthcareautomated tumor delineation in radiation therapydeep learning for cancer treatmentdynamic lung tumor trackingenhancing patient outcomes with AIimproving accuracy in radiation therapyinnovations in cancer treatment technologyovercoming challenges in cancer treatment planningprecision mapping of lung tumorstumor segmentation using artificial intelligence