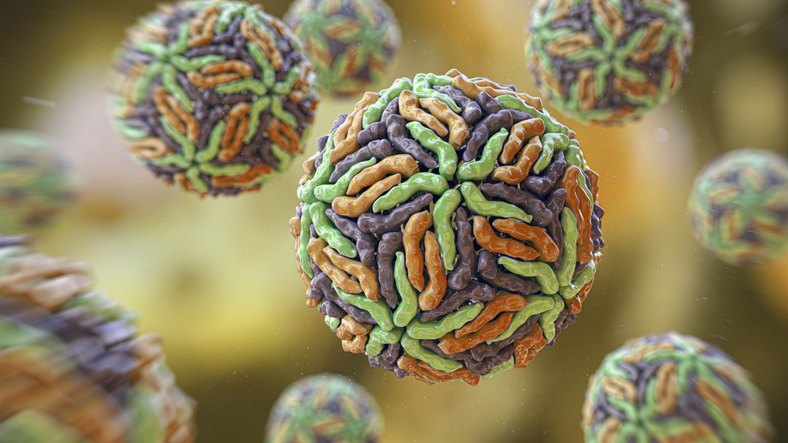
Zika virus (ZIKV) is a mosquito-borne orthoflavivirus that can cause birth defects, including microcephaly, a condition in which head and brain size are significantly reduced at birth. The collective results of the newly reported study involving work in human cell lines, fruit flies, and mosquito cells, have shown how Zika virus hijacks a host protein called ANKLE2, which is important for brain development, to assist its own replication.
The new work found that related viruses, including dengue virus and yellow fever virus, also use ANKLE2 for the same purpose. But because Zika—unlike most related viruses—can cross the placenta, Zika virus infection can have disastrous consequences in pregnancy. “It’s a case of Zika being in the wrong place at the wrong time,” said Priya Shah, PhD, associate professor in the departments of microbiology and molecular genetics and of chemical engineering at the University of California, Davis. The discovery could point to new strategies for developing vaccines or therapeutics against Zika and related viruses.
Shah is senior author of the team’s published paper in mBio, titled “Microcephaly protein ANKLE2 promotes Zika virus replication.” In the paper, the team concluded that in combination with their previous results, the new findings “… indicate that ZIKV and other orthoflaviviruses hijack ANKLE2 for a conserved role in replication, and this drives unique pathogenesis for ZIKV since ANKLE2 has essential roles in developing tissues.”
Orthoflaviviruses are positive-sense single-stranded RNA viruses that cause severe disease, the authors wrote. “Many orthoflaviviruses, such as Zika virus (ZIKV), dengue virus (DENV), West Nile virus (WNV), and yellow fever virus (YFV), are transmitted by mosquitoes and represent significant public health threats worldwide.”
Viruses carry only a limited set of instructions in their own genetic material, so to replicate they rely on taking over host cell proteins and functions. Shah’s laboratory studies these interactions between virus and host.
A Zika virus epidemic across South and Central America in 2015 led to the emergence of the virus as a global threat, the team explained. While infection with Zika virus in adults typically leads to mild symptoms and very rarely Guillain-Barré syndrome, “the primary concern surrounding ZIKV arises from the occurrence of congenital Zika syndrome (CZS) in individuals infected in utero,” they noted. “CZS is a spectrum of disease and can be clinically characterized by multiple hallmark features, including congenital contractures, ocular anomalies, cortical calcifications, and in the most severe cases, microcephaly,” a condition that is linked to a range of complications, including developmental delays, intellectual disability, and predisposition to seizures.
The team had previously found that a Zika virus protein called NS4A interacts with ANKLE2 in host cells. “By searching for host proteins with known roles in neurodevelopment or associations with microcephaly, we identified the interaction between ZIKV NS4A and host ankyrin repeat and LEM domain-containing 2 (ANKLE2),” they wrote in their newly published paper. Working in Drosophila, the team had shown that this could lead to microcephaly. “Using this Drosophila model, we previously showed that transgenic expression of ZIKV NS4A induces similar microcephaly phenotypes which are also rescued by the expression of human ANKLE2.”
ANKLE2 is known to be involved in brain development in the fetus but is found in cells throughout the body. For their newly reported study, led by recent PhD graduate Adam Fishburn, Shah’s team grew Zika virus in human cells. They found that ANKLE2 gene knockout (KO) in these cells reduced the ability of Zika virus to grow.
Viral NS4A interacts with ANKLE2 to form pockets off the endoplasmic reticulum that act as virus factories, Shah said. Bringing all the components to make viruses in one place makes replication more efficient and also helps hide the virus from the immune system. In Zika-infected cells, ANKLE2 clusters in pockets around the endoplasmic reticulum, a network used for protein production within the cell. “Microscopy suggested that ANKLE2 KO results in fewer and poorly formed virus replication organelles and significantly altered membrane rearrangements,” they wrote. “Our current model is that ANKLE2 is really important, but not essential, for forming these replication pockets,” Shah commented.
Our cells are well equipped to fight off these viruses, but only if they can find them, Fishburn added. “Zika and related viruses have evolved strategies to hide themselves in these replication pockets to avoid detection.” The authors suggested that their study “… supports a model in which ANKLE2 promotes virus-mediated ER remodeling to accelerate and conceal genome replication.” Fishburn added, “We believe that ANKLE2 is hijacked to help facilitate this process, and without it the pockets don’t form as well and the immune system can keep virus replication in check.”
Working with Claudia Rückert, PhD, at the University of Nevada, the investigators found that Zika virus also uses ANKLE2 when it infects mosquito cells, meaning that this interaction is important in both human and insect hosts. “Furthermore, we show that silencing of the ANKLE2 ortholog in mosquito cells also reduces ZIKV replication, suggesting a conserved role in replication across hosts,” they stated.
Finally, they showed that NS4A from other, related mosquito-borne viruses, including dengue virus and yellow fever virus, interacts with ANKLE2 in the same way. “… we show that NS4A from four other orthoflaviviruses physically interacts with ANKLE2 and is also beneficial to their replication.” This all suggests that NS4A/ANKLE2 interaction is important for replication across a broad group of mosquito-borne viruses, opening possible routes to new drugs and vaccines for these diseases. “Our data point to an overall model in which ANKLE2 regulates virus-induced membrane rearrangements to accelerate orthoflavivirus replication and avoid immune detection,” the team stated.
If much more common viruses like dengue virus also target ANKLE2, why don’t they also cause the microcephaly seen in Zika virus infection? It’s probably all down to location. Zika virus is unusual in that it can cross the placenta and enter the fetus, where ANKLE2 is known to play a big role in brain development. Most other viruses are kept out of the fetus by the placental barrier. “Thus, ZIKV’s unique pathogenesis derived from the NS4A-ANKLE2 interactions is from being in the wrong place at the wrong time when it comes to ANKLE2,” the scientists concluded in their paper.