New research reveals groundbreaking insights into male infertility, shedding light on the role of a critical gene, transducin-like enhancer of split 6 (TLE6). Conducted by a team from Kanazawa Medical University in Japan, the study focuses on a novel hetero knockout mouse model specifically designed to investigate how deficiencies in the TLE6 gene affect male reproductive health. This study is paramount, as infertility poses significant psychological and physiological challenges globally, and understanding its genetic underpinnings is vital for developing targeted therapies.
TLE6 is a crucial member of the subcortical maternal complex (SCMC), a multi-protein complex essential for proper embryo development and cell division. Researchers have established that mutations in SCMC-related genes can lead to severe reproductive issues in females. However, the role of these mutations in male infertility has remained largely unexplored until now. This innovative study not only highlights male fertility challenges but also investigates the implications of TLE6 deficiency in sperm development and function.
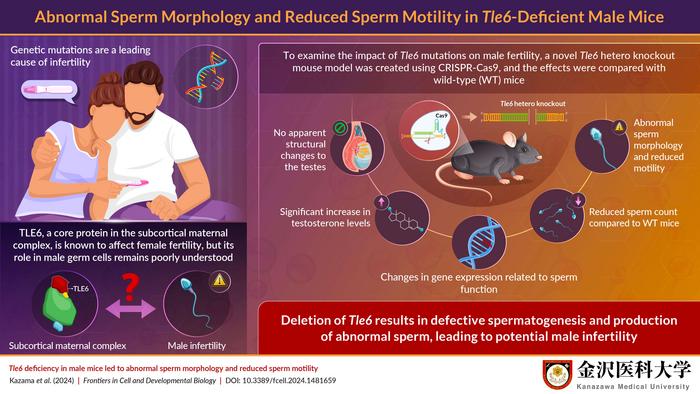
The research team, led by Mr. Kousuke Kazama, employed state-of-the-art CRISPR-Cas9 technology to create male mice with heterozygous deletions of the Tle6 gene. This gene-editing technique allows for precise alterations in DNA sequences, paving the way for a clearer understanding of gene function and its impacts on biological systems. The team’s findings were recently published in the peer-reviewed journal “Frontiers in Cell and Developmental Biology,” making significant inroads into a previously uncharted area of reproductive biology.
Initial experiments involved studying mating behavior between Tle6-deficient male mice and wild-type counterparts. Surprisingly, the mating frequency and the number of offspring produced were comparable between the two groups. This perplexing result led researchers to contemplate the potential reasons behind the lack of inheritance of Tle6 deficiency traits into the next generation. Further analysis directed their attention to sperm quality, compelling them to delve into the structural and functional characteristics of sperm produced by Tle6-deficient males.
Scrutinizing the morphology of sperm revealed concerning abnormalities. Notably, a staggering 57% of spermatozoa displayed atypical head structures, while 7% exhibited double-headed formations. Such defects in sperm morphology could critically impair fertilization capabilities, drawing attention to the vital role that TLE6 plays in ensuring the proper development of viable sperm.
Complementing these findings, the researchers observed noteworthy alterations in hormone levels in Tle6-deficient mice. Elevated testosterone levels were discovered, raising questions about the potential dysregulation of endocrine signals associated with TLE6 deficiency. The interconnectedness of hormonal balance and reproductive health cannot be overstated, as hormones are vital for the regulation of numerous physiological processes, including sperm production and maturation.
To further understand the functional implications of TLE6 deficiency, researchers employed immunofluorescence staining to visualize the localization of the TLE6 protein within sperm cells. This revealed a surprising overlap between TLE6 localization and the midpiece of spermatozoa, an area rich in mitochondria, the powerhouse of the cell. This finding suggests that TLE6 may be involved in energy production crucial for sperm motility – a key factor in successful fertilization.
In conjunction with structural assessments, gene expression analyses of testes from Tle6-deficient mice highlighted an intriguing increase in genes associated with fertilization, sperm motility, and structural integrity. These observations collectively underline how TLE6 deficiencies disrupt normal sperm development while simultaneously propelling alterations in gene expression that may pose further ramifications on overall fertility health.
The research holds significant implications for clinical practices regarding male infertility. As rates of infertility continue to rise, understanding the genetic factors contributing to this condition enables healthcare professionals to develop targeted interventions, whether through gene therapies or enhanced diagnostic tools. The ultimate goal is to provide couples facing infertility challenges with more effective treatment options based on genetic insights.
As the study progresses, researchers emphasize the importance of translating these findings from mouse models to human fertility. While mice serve as excellent models due to their genetic similarities and controlled breeding environments, the translation of these results to human biology necessitates careful consideration. Future studies will need to elucidate the variations in TLE6’s role in humans and mice, guiding further investigations into potential therapies for those suffering from male infertility.
The Kanazawa Medical University research team is hopeful that their findings will act as a catalyst for more expansive studies that could unveil additional aspects surrounding male fertility. With ongoing research focused on the intricate relationships between genetics, environmental factors, and reproductive health, there is optimism for the future treatment of infertility disorders.
As we advance toward more personalized medicine approaches, discoveries like these pave the way for individualized treatment plans that take into account a patient’s unique genetic makeup. This study exemplifies how molecular genetics can unlock the mysteries surrounding reproduction and fertility, offering hope to many families longing to conceive.
In conclusion, the revelations surrounding TLE6’s impact on male fertility illuminate a significant and underexplored facet of reproductive biology. With growing awareness and understanding, the potential for more comprehensive reproductive health solutions continues to expand, promising a brighter future for those affected by infertility.
Subject of Research: Animals
Article Title: Tle6 deficiency in male mice led to abnormal sperm morphology and reduced sperm motility
News Publication Date: 24-Oct-2024
Web References: [Not available]
References: [Not available]
Image Credits: Credit: Kousuke Kazama from Kanazawa Medical University
Keywords: Infertility, Sperm, Genetic structure, Protein structure, Testicles, Male fertility, Clinical research, Biomedical research funding.