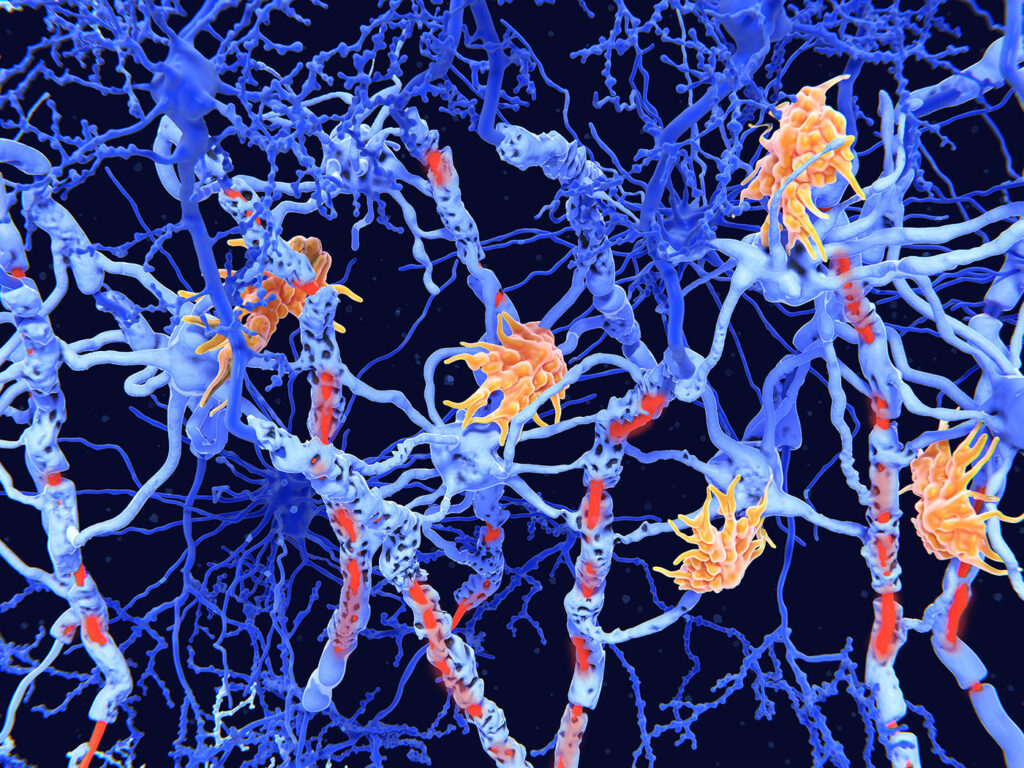
Scientists using “disease in a dish” technology to study multiple sclerosis (MS) identified an unusual type of brain cell that they say may play a vital role in progressive multiple sclerosis (PMS), likely contributing to the persistent inflammation characteristic of the disease.
The researchers, headed by teams at University of Cambridge, U.K., and at the National Institute on Aging, suggest their discovery of the disease-associated radial glial-like (DARG) cells represents a significant step towards understanding the complex mechanisms that drive MS and provides a promising new avenue for research into more effective therapies.
Research co-lead Professor Stefano Pluchino, MD, PhD, at the Department of Clinical Neurosciences at the University of Cambridge, said, “Progressive MS is a truly devastating condition, and effective treatments remain elusive. Our research has revealed a previously unappreciated cellular mechanism that appears central to the chronic inflammation and neurodegeneration driving the progressive phase of the disease.”
Pluchino and colleagues reported on their findings in Neuron, in a paper titled “Integrated omics reveals disease-associated radial glial-like cells with epigenetically dysregulated interferon response in multiple sclerosis.” In their paper the authors concluded, “DARGs may sustain smoldering inflammation, unveiling a previously unrecognized cellular axis that could underpin mechanisms in neurodegeneration. This discovery offers novel insights into disease mechanisms and highlights potential therapeutic targets.”
MS is a chronic disease in which the immune system mistakenly attacks the brain and spinal cord, disrupting communication between the brain and the body. While many individuals initially experience relapses and remissions, a significant proportion transition to progressive MS, a phase marked by a steady decline in neurological function with limited treatment options. “Progressive multiple sclerosis (PMS) involves a persistent, maladaptive inflammatory process with numerous cellular drivers,” the authors wrote.
To model what is happening in the disease, the researchers took skin cells from patients with progressive MS and reprogrammed them into induced neural stem cells (iNSCs), an immature type of cell capable of dividing and differentiating into various types of brain cells. Importantly, the direct reprogramming technology preserved epigenetic memory of the donor cells, they commented. “We generated induced neural stem cells (iNSCs) from patient fibroblasts through a direct reprogramming protocol that preserved their epigenome …”.
Using this ‘disease in a dish’ approach, the team observed that a subset of the cultured brain cells was somehow reverting to an earlier developmental stage, transforming into an unusual cell type known as radial glia-like (RG-like) cells. Notably, these cells were highly specific and appeared approximately six times more frequently in iNSC lines derived from individuals with progressive MS, compared to controls. These cells were designated as disease-associated RG-like cells (DARGs).
These DARGs exhibit characteristic features of radial glia—specialized cells that serve as scaffolding during brain development and possess the capacity to differentiate into various neural cell types. Essentially, they function both as structural support and as fundamental building blocks, making them critical for proper brain development. Unexpectedly, the team found DARGs not only reverted to an ‘infant’ state but also displayed hallmark features of premature aging, or senescence. “Our characterization revealed that PMS-derived iNSCs recapitulate key features of the disease, including elevated inflammatory signaling and senescence gene expression, aligning with prior reports,” the investigators wrote.
These identified DARGs also possessed a distinctive epigenetic profile—patterns of chemical modifications that regulate gene activity—although the factors influencing this epigenetic landscape remain unclear. These modifications contribute to an exaggerated response to interferons (IFNs), the immune system’s ‘alarm signals,’ which may help explain the high levels of inflammation observed in MS.
“Our study reveals epigenetic changes in somatic fibroblast isolated from people with PMS, retained after direct reprogramming into iNSCs,” the authors stated. “Comprehensive transcriptomic profiling confirmed increased RNA expression of senescence, inflammation, and IFN signaling pathways in PMS-derived iNSCs driven by IFN-associated transcription factors (TFs).” Pluchino added, “Essentially, what we’ve discovered are glial cells that don’t just malfunction—they actively spread damage. They release inflammatory signals that push nearby brain cells to age prematurely, fueling a toxic environment that accelerates neurodegeneration.”
The team validated the findings by cross-referencing with human data from individuals with progressive MS. By analyzing gene expression patterns at the single-cell level—including new data exploring the spatial context of RNA within post-mortem MS brain tissue—they confirmed that DARGs are specifically localized within chronically active lesions, the regions of the brain that sustain the most significant damage. “Corroborating analyses of post-mortem single nucleus and spatial transcriptomics datasets identified a distinct, non-neurogenic, disease-associated RG-like cell population (DARGs) within chronic active lesions with the potential to fuel smoldering inflammation in PMS,” they state. Importantly, DARGs were found near inflammatory immune cells, supporting their role in orchestrating the damaging inflammatory environment characteristic of progressive MS.
By isolating and studying these disease-driving cells in vitro, the researchers aim to explore their complex interactions with other brain cell types, such as neurons and immune cells. This approach will help to explain the cellular crosstalk that contributes to disease progression in progressive MS, providing deeper insights into underlying pathogenic mechanisms. In conclusion, the team stated, “…our integrated in vitro and tissue analyses reveal DARGs, which display hallmark features of inflammation and senescence,” the scientists noted. “Their enrichment in chronic lesions and association with ongoing neurodegeneration suggest that targeting this cellular axis may provide new avenues for therapeutic intervention aimed at disrupting disease progression in PMS.”
Alexandra Nicaise, PhD, co-lead author of the study, Department of Clinical Neurosciences at Cambridge, added: “We’re now working to explore the molecular machinery behind DARGs, and test potential treatments. Our goal is to develop therapies that either correct DARG dysfunction or eliminate them entirely. If we’re successful, this could lead to the first truly disease-modifying therapies for progressive MS, offering hope to thousands living with this debilitating condition.”
To date, DARGs have only ever been seen in a handful of diseases, such as glioblastoma and cerebral cavernomas, clusters of abnormal blood vessels. However, this may be because scientists have until now lacked the tools to find them. Professor Pluchino and colleagues believe their approach is likely to reveal that DARGs play an important role in other forms of neurodegeneration.