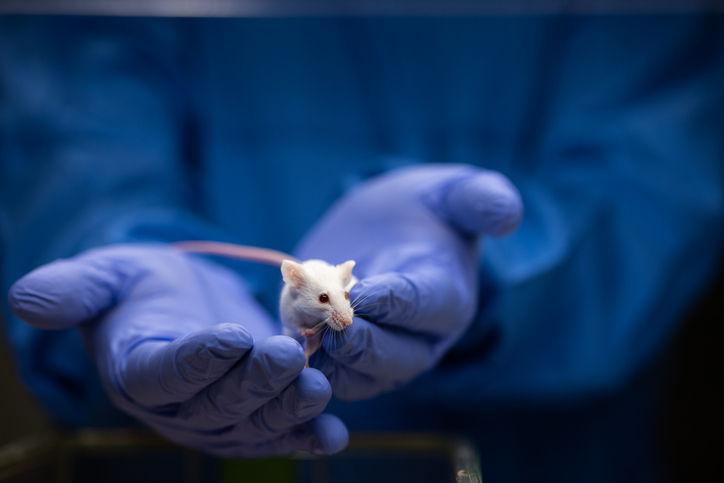
Researchers at Washington State University (WSU) report that they have created genetically-engineered mice that could help accelerate anti-aging research.
“This is the first mouse model with truly humanized telomeres because telomerase isn’t expressed in adult tissues in this model,” said Jiyue Zhu, PhD, professor, college of pharmacy and pharmaceutical sciences. “Our paper demonstrates that they exhibit human-like telomeres. Now, we aim to observe how these mice age.”
The team’s study Modification of the telomerase gene with human regulatory sequences resets mouse telomeres to human length appears in Nature Communications.
Globally, scientists are working to unlock the secrets of extending human lifespan at the cellular level, where aging occurs gradually due to the shortening of telomeres. As telomeres shorten over time, cells lose their ability to divide for healthy growth, and some eventually begin to die.
HuT mice
But research studying these telomeres at the cellular level has been challenging in humans, according to Zhu, whose team was successful in developing mice that have human-like short telomeres, enabling the study of cellular aging as it occurs in the human body and within organs. Normally mice have telomeres that are up to 10 times longer than humans.
Called HuT mice for their humanized telomeres, they are enabling Zhu’s team to advance multiple research projects. Key areas of focus include studying how short telomeres reduce the likelihood of developing cancer and influence human lifespan, as well as exploring strategies to extend individuals’ health span—the period of life free from age-related diseases.
The work has significant implications for the development of future drugs and treatments, noted the scientists. In the long term it may pave the way for anti-aging strategies aimed to activating cells to protect telomeres and potentially extend lifespans. Zhu pointed out that many diseases originate at the cellular level, so targeting drugs there is a common strategy.
Telomerase levels are crucial because cancer cells divide rapidly and need high amounts of telomerase to maintain their telomeres.
“One of our goals is to reduce telomerase expression in cancer cells, and this is an active area of research,” continued Zhu, adding that the mouse model allows for multiple aging studies.
Sleep and human health
One of his collaborators, Christopher Davis, PhD, WSU Elson S. Floyd College of Medicine, studies how sleep impacts human health. The group will use HuT mice to see how the stress of sleep deprivation and other life stresses affect telomere regulation and aging.
Zhu began telomere studies in the mid-1990s under researchers and Nobel Prize winners Elizabeth Blackburn, PhD, and J. Michael Bishop, MD, both pioneers in understanding telomeres and cancer. Zhu joined WSU in 2014.
The development of HuT mice began 10 years ago, when he and other researchers gained a deeper understanding of telomere regulation in humans and how it differs from animals. Previously, how telomeres regulated the human aging process could only be studied using isolated human cells in a petri dish.
“This mouse model is quite different, as it allows us to observe the aging process in a whole organism,” Zhu said. “Mice are similar to humans in terms of organ structure, genes, and genetic makeup.”
The WSU team hopes to eventually share the mice with other research teams to help advance studies on aging, human longevity, and cancer. Zhu received $5 million in grants for studies to further develop the mouse model that simulates human replicative aging as well as to research cancer implications.