Targeted protein degradation is a therapeutic strategy that utilizes the cell’s natural machinery to eliminate disease-causing proteins. The field traces its origins to the development of thalidomide in the 1950s, although the mechanisms of action were initially unclear. Originally used to treat nausea in pregnant women, thalidomide was later shown to cause severe congenital disabilities when used during pregnancy. Since then, newer generations of targeted protein degraders—such as proteolysis-targeting chimeras (PROTACs) and molecular glue degraders—have shown enhanced safety and efficacy. We spoke with leaders from four top companies to explore the current state of this rapidly evolving field.
PROTACs await first pivotal data
Randy Teel, PhD, chief business officer of Arvinas, explains how PROTACs differ from traditional drugs. “Traditional drugs tend to block or inhibit a protein of interest. But they don’t get rid of the protein. They just prevent it from doing what it is supposed to do.”
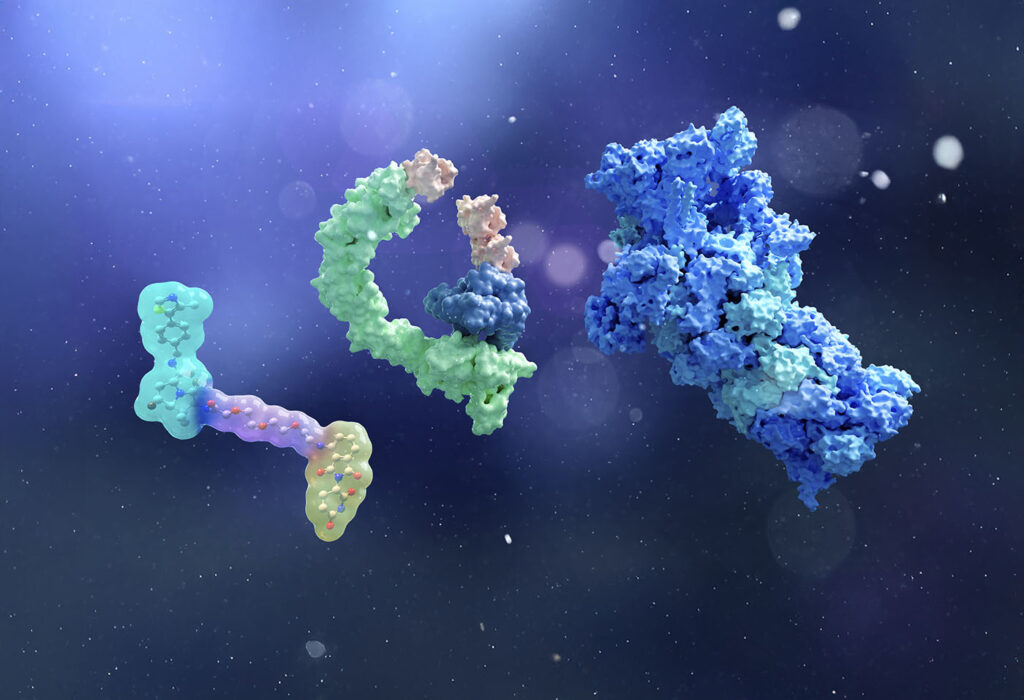
In contrast, a PROTAC directs the body’s natural degradation mechanisms to eliminate the entire disease-related protein. “Your body already degrades protein. With PROTACs, we are using a small molecule drug to harness that natural machinery,” notes Teel. He explains that a PROTAC is a small molecule with two ends (Figure 1). One end binds to the target protein of interest, while the other binds to the cellular machinery responsible for protein degradation. In the case of a PROTAC, this binding involves an E3 ligase.

Why would a PROTAC be better than traditional drugs? Teel explains that PROTACs involve an iterative mechanism in which one copy of the PROTAC can lead to the degradation of hundreds of copies of the target. Theoretically, this implies higher potency with lower doses.
The second advantage is that PROTACs can be used against so-called undruggable targets. Teel notes that many proteins are undruggable because no small molecule has been found that can bind tightly to them. “But we don’t need to bind tightly with a PROTAC. We just need to bind long enough to enable the degradation process,” he explains.
Although no PROTACs are currently approved for clinical use by the FDA, Teel highlights the company’s current and upcoming Phase III trials for vepdegestrant (ARV-471), an estrogen receptor degrader designed to treat breast cancer. The first trial involves PROTAC monotherapy for breast cancer patients in the second-line-plus setting. Initial results are expected in the first quarter of 2025.
Teel also notes the company’s two other ongoing Phase I trials. One is using ARV-393, a PROTAC for blood cancers that degrades BCL6, a previously undruggable target. The second involves ARV-102, which degrades a protein relevant to neuroscience.
“Our neuroscience application is a big deal because it is the first PROTAC I know of that has been specifically targeted at a central nervous system protein. We expect to demonstrate that we can get through the blood-brain barrier,” Teel adds.
Degrading extracellular proteins
Extracellular proteins, which comprise about 40% of all proteins, cannot easily be targeted by PROTACs, notes Ann Lee-Karlon, PhD, CEO and president of EpiBiologics. She highlights that EpiBiologics is building drugs that can selectively degrade membrane proteins.
Lee-Karlon explains how one arm of the company’s EpiTAC molecules binds to the disease-causing target while the other binds to a degrading receptor (Figure 2). Ultimately, the goal is to drive extracellular protein degradation through either the proteasome or lysosome.

Unlike PROTACs—which use small molecules to degrade proteins inside cells—EpiTAC antibodies can target proteins located outside of the cell. Therefore, EpiTACs need not be optimized for cell permeability. And while PROTACs are not specific to tissues, EpiTACs localize the degradation to the diseased site. “Our main differentiator is selectivity,” says chief scientific officer Shyra Gardai, PhD. “We can be tissue-selective, cell-selective, or disease-selective.”
“The key with our technology is that we localize degradation to just the disease area, sparing healthy tissue and increasing efficacy,” she adds. “That is very important for targets like EGFR. With our technology, we can spare healthy EGFR in the skin and colon and just degrade EGFR in the tumor,” says Gardai.
Furthermore, EpiBiologics has an extensive receptor repertoire with over 270 different degrading receptors. “From this collection, we can choose the degrader to partner with the target of interest to remove the protein and scaffolding,” she notes. Promisingly, EpiTACs can also degrade G protein-coupled receptors, which are typically considered undruggable.
The company’s lead program is a tissue-selective bispecific antibody driving EGFR degradation for solid tumors. Preclinical studies using it as a monotherapy in mice have demonstrated tumor shrinkage across various tumor types, including those with resistance mechanisms to standard of care. The drug’s activity is better than current clinical benchmarking molecules, Gardai says, and no overt safety signals have been observed.
“Our goal in the coming year is to prepare our EGFR degrader to enter the clinic and to progress our pipeline in both oncology and immunology,” Lee-Karlon says. “We anticipate future clinical trials in lung cancer and other solid tumors.”
Degrading proteins with light
Nathan Tague, PhD, cofounder of SynsoryBio, cites his previous research in optogenetic degradation, which involves degrading proteins with light. He explains that most proteins, especially those in organisms that are not photosynthetic, do not respond to light naturally. “However, protein domains from plants and light-responsive microbes can be synthetically incorporated into organisms that normally don’t respond to light. In this way, proteins can be engineered to respond to light,” he says.
The advantage of using light as a medium to direct cell function boils down to the ability to control light in time (by turning the light source on and off) or in space (by limiting light exposure to specific regions). Tague notes that there has been an explosion in optogenetic tools—some of which work in humans—to direct cell behavior over the past decade.
Tague’s research involves the LOVdeg tag,1 which is derived from a protein domain in the common oat that has been mutated to interface with bacterial proteosomes. This modular domain can be fused to any protein of interest that researchers want to control with light, he notes.
In the dark, the LOVdeg tag exists in a closed state and does not affect the stability of the protein with which it is fused. However, once exposed to blue light, the tag changes shape. “In the presence of light, the LOVdeg tag becomes unstructured and proteases will grab onto it and degrade it along with the fused protein,” he explains.
The LOVdeg tag is currently only designed for use in bacteria. Tague lists various potential applications, including synthetic biology, bioreactors, and microbiome therapeutics.
While optogenetic targeted protein degradation has not yet been optimized in the clinical setting for therapeutic agents, Tague envisions that this modality may become more popular in the future. “You can imagine wanting to achieve targeted degradation with higher precision as the field develops and if systemic degradation causes unwanted side effects,” he says. “In this case, the added layer of light activation for spatial or temporal control would be a compelling next step.”
However, he also notes that a practical challenge would involve the delivery of the light, which would have to be achieved through tissue-penetrating wavelengths of light or endoscopic methods.
Unveiling PROTAC kinetics
Judith Ronau, PhD, a senior scientist in Biochemistry at AbbVie, and Lake Paul, PhD, the CEO of BioAnalysis, discussed an ongoing collaboration between the two organizations to understand the kinetic properties of PROTACS (work being published in an upcoming issue of the European Biophysics Journal). They explain their use of analytical ultracentrifugation (AUC) with a specialized ultracentrifuge with optics to enable the live monitoring of macromolecules in solution under biologically relevant conditions (Figure 3).

[Alex Yarawksy, BioAnalysis]
“At AbbVie, we are committed to understanding the mechanism of degrader action through the development of assays designed to characterize each step of the process,” explains Ronau. She notes that information garnered from these studies aids the company in ranking the potential effectiveness of different degrader compounds.
Overall, AUC is a convenient and efficient method that can provide information about everything from the presence of aggregates to reaction kinetics, conformational changes, and the relative abundance of different complexes. Ronau emphasizes that the new platform provides key data about PROTAC complexes in their native state.
Paul notes that analyzing these parameters often requires several experimental techniques—including native mass spectrometry, size-exclusion chromatography, surface plasmon resonance, immunoassays, calorimetry, and bioluminescence resonance energy transfer methods.
Ronau stresses that a critical phase in PROTAC kinetics involves ternary complex formation, during which the degrader mediates the interaction between the targeted protein and the degrader (e.g., an E3 ubiquitin ligase). “While the broader degrader community has developed various assays for this step of the process, AUC has been overlooked,” she says.
“Ultimately, this AUC platform has the potential to speed up the experimental timelines in the targeted protein degrader space while simultaneously reducing cost and labor,” says Lake.
The future of PROTACs
“With the first pivotal data in 2025, I think this could be the year where we really get rid of any doubt that PROTACs are going to be a technology that works,” concludes Teel. “Neuroscience will be an important frontier, but there will be a lot of frontiers beyond that.”
Reference
- Tague N, Coriano-Ortiz C, Sheets MB, Dunlop MJ. Light-inducible protein degradation in E. coli with the LOVdeg tag. Elife. 2024 Jan 25;12:RP87303. doi:10.7554/eLife.87303.