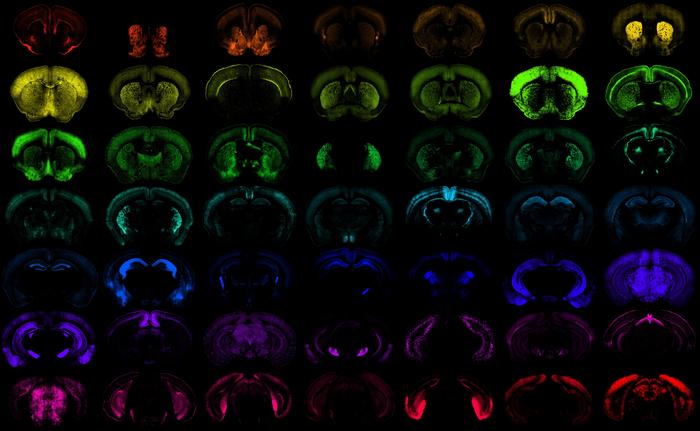
The mammalian cortex, the region of the brain responsible for a wide range of higher-level cognitive functions, including sensory perception, motor control, and complex thought, is composed of a diverse array of cell types. Defining the contribution of each cell type to cortex function is an ongoing challenge for understanding brain health and disease.
In a new study published in Cell titled “A suite of enhancer AAVs and transgenic mouse lines for genetic access to cortical cell types,” researchers from around 29 universities and institutions across North America have developed a new suite of genetic tools for cell-type specific targeting to battle brain disease.
According to Bosiljka Tasoc, PhD, director of molecular genetics at the Allen Institute and corresponding author of the study, diseases usually arise from flaws in specific cell types, not the whole organism. As an example, Tasoc stated that epilepsy is a disease that targets specific neurons in the nervous system.
“If you want to fix those neurons, you can try to access only those neurons. The key is this cell-type specific access for understanding and perturbing brain cells to figure out their function, and for correcting and fixing the defective parts of these cells,” said Tasoc.
Researchers used transcriptomic and epigenomic cell-type taxonomies from mice and humans to define marker genes and putative enhancers and create a large toolkit of transgenic lines and enhancer adeno-associated viruses (AAV) vectors that carry DNA sequences to trigger selective targeting of cortical cell populations. The authors generated and evaluated over 1,000 different enhancer AAV vectors covering most subclasses of cortical cells.
This new arsenal of enhancer AAVs opens the door to targeted gene therapies that can correct genetic defects in specific cells implicated in disease without affecting surrounding cells and causing unwanted side effects.
“The rapidly growing collection of brain cell type enhancer AAV vectors with unprecedented strength and specificity of labeling hold great promise to enable new avenues for brain cell type targeting and perturbation in diverse mammalian model organisms and potentially humans,” said Jonathan Ting, PhD, one of the study authors and associate investigator at the Allen Institute.
The collective findings using this enhancer AAV toolbox were published in eight studies in the Cell Press family of journals, including Neuron, Cell, Cell Reports Methods, Cell Reports, and Cell Genomics. Collaborators include the Allen Institute, Broad Institute, Harvard Medical School, Duke University, University of California, Irvine, University of California, Berkeley, University of Pittsburg, Carnegie Mellon, Stanford, University of Washington, and Addgene.
Among the brain cell type portfolio are the successful enhancer AAV design for both cortex and striatum brain regions, as well as the spinal cord.
The work is part of the Armamentarium for Precision Brain Cell Access, a project within the National Institutes of Health’s (NIH) Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative, whose central goal is to develop, scale, and distribute a comprehensive toolkit, termed an “armamentarium,” of molecular and genetic tools that can interface with specific brain cells.
“Homing in on the right cells—in the right way and at the right time—is the future of precision brain medicine,” said John Ngai, PhD, director of the NIH BRAIN Initiative. “These tools move us closer to that future, while also expanding what we know about the brain’s cells and circuits today.”
The tools and data in the studies are freely available on the Allen Institute’s Genetic Tools Atlas and through Addgene.