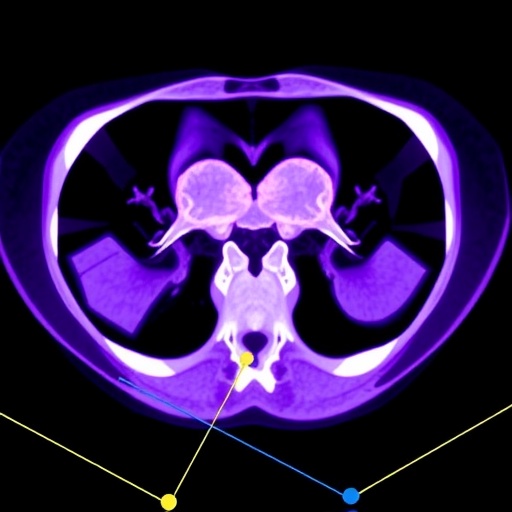
In a groundbreaking advancement for cancer diagnostics, researchers have developed an innovative deep learning algorithm designed to revolutionize the way endometrial cancer is analyzed through medical imaging. This study, recently published in BMC Cancer, harnesses the power of ^18F-FDG PET/CT scans combined with a novel segmentation approach to better understand genetic expression patterns in tumors. The research illuminates the intricate relationship between imaging features and gene mutations, offering a potent new tool in personalized cancer treatment and prognosis.
The research team focused on creating an automated segmentation method tailored specifically for PET/CT images of endometrial cancer. Segmentation, the process of delineating tumor boundaries from medical images, has historically been a labor-intensive, variable task prone to inconsistency. Traditional methods often rely on manual annotation or simpler computational models, which cannot adequately capture the complex spatial and metabolic heterogeneity of tumors. By integrating a deep learning-based PET Attention-UNet architecture, the team elevated segmentation accuracy, effectively separating cancerous tissue with unprecedented precision.
This deep learning approach led to remarkable performance metrics, boasting a dice coefficient — a statistical measure of overlap between predicted and true tumor areas — exceeding 97% on training data and maintaining strong accuracy during validation. Such levels of precision signify an exceptional ability to localize tumors, which is critical for subsequent analyses. These improvements in segmentation pave the way for extracting reliable radiomic features that serve as the foundation for predictive modeling of gene expression.
.adsslot_54bDJqnE9R{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_54bDJqnE9R{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_54bDJqnE9R{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Radiogenomics, the emerging interdisciplinary field that marries imaging phenotypes with genomic data, stands at the heart of this study. The investigators directed their efforts toward predicting the expression of two pivotal genes implicated in endometrial cancer progression: Mismatch Repair (MMR) genes and TP53. MMR gene status is vital for determining prognosis and guiding immunotherapy decisions, while TP53, often dubbed the “guardian of the genome,” is a critical tumor suppressor whose mutations promote malignancy. Traditionally, assessing these genes required invasive procedures; this study demonstrates that non-invasive imaging can predict gene expression profiles with respectable accuracy.
By harnessing datasets comprising hundreds of patients with confirmed endometrial cancer, the researchers trained and tested radiomics models built on PET, CT, and combined PET+CT imaging features. It emerged that the integrative model leveraging both PET’s metabolic insights and CT’s anatomical details consistently outperformed models limited to a single modality. This synergistic information enhanced the ability to forecast MMR status and TP53 mutations, revealing nuanced tumor heterogeneity linked to genetic alterations.
The analysis revealed that the phenotypic heterogeneity detected by PET imaging correlated strongly with variations in MMR-related protein expression, suggesting metabolic activity as a window into gene-driven tumor behavior. TP53 expression differences were also predominantly observable through PET features, emphasizing the scan’s utility in capturing functional aberrations beyond the physical tumor architecture highlighted by CT. These findings underscore the importance of a multimodal imaging approach for comprehensive tumor characterization.
This novel segmentation and radiomics framework not only automates and refines the tumor detection process but also translates complex image data into actionable molecular information. The predictive performance was quantified using the area under the receiver operating characteristic curve (AUC), with values reaching beyond 0.8 for both MMR and TP53 prediction. Such metrics point to clinical relevance, as models achieving this level of accuracy can potentially assist oncologists in stratifying patients for targeted therapies or follow-up regimens without necessitating repeated biopsies.
The integration of deep learning into radiogenomics represents a significant stride toward precision oncology, where treatment plans are tailored to individual tumor biology. Endometrial cancer, a disease where early and accurate characterization can drastically influence outcomes, stands to benefit immensely. The improved efficiency and reliability brought forth by this technology could reduce diagnostic turnaround times and minimize subjectivity inherent in pathology.
Moreover, the study’s methodology illustrates a scalable approach that could be adapted to other cancers and imaging modalities. The use of a retrospective, exploratory design allowed for robust model development using existing clinical datasets, highlighting the potential for rapid deployment in diverse healthcare settings. Attention mechanisms embedded in the UNet architecture further enhance the model’s capacity to focus on critical imaging regions, improving interpretability and performance.
The researchers emphasize that while the algorithm’s segmentation capabilities are near state-of-the-art, these tools complement rather than replace traditional diagnostic methods. Integration with clinical workflows requires ongoing validation and the development of user-friendly interfaces. Yet, the promise of combining imaging-derived phenotypes with molecular data signals a new era where non-invasive, image-based diagnostics can guide personalized cancer management with unprecedented precision.
Looking ahead, expanding patient cohorts, incorporating longitudinal data, and integrating additional omics layers such as transcriptomics or proteomics could amplify the predictive power and scope of such models. Additionally, leveraging explainable AI techniques may foster greater clinician trust, elucidating how specific imaging features relate to gene expression patterns, and unlocking further biological insights.
In summary, this cutting-edge research represents a paradigm shift in the intersection of medical imaging, artificial intelligence, and cancer genomics. By developing an advanced deep learning segmentation pipeline and demonstrating its application in radiogenomic prediction, the study offers a powerful tool to decode the genetic underpinnings of endometrial tumors via PET/CT scans. The convergence of these technologies heralds a future where imaging biomarkers not only visualize tumors but also reveal their molecular identities, ultimately leading to more precise, effective, and personalized cancer care.
Subject of Research: Radiogenomics study integrating ^18F-FDG PET/CT imaging and deep learning segmentation to predict MMR and TP53 gene expression in endometrial cancer.
Article Title: A radiogenomics study on ^18F-FDG PET/CT in endometrial cancer by a novel deep learning segmentation algorithm
Article References: Li, X., Shi, W., Zhang, Q. et al. A radiogenomics study on ^18F-FDG PET/CT in endometrial cancer by a novel deep learning segmentation algorithm. BMC Cancer 25, 1006 (2025). https://doi.org/10.1186/s12885-025-14392-6
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14392-6
Tags: ^18F-FDG PET/CT scansaccuracy in tumor delineationautomated segmentation methods in oncologyBMC Cancer publication on endometrial cancercancer diagnostics advancementscancer prognosis and treatmentcomplex spatial heterogeneity in tumorsdeep learning in medical imagingmachine learning algorithms in healthcarepersonalized cancer treatment approachesPET/CT analysis for endometrial cancertumor genetic expression patterns