In a significant advancement in our understanding of lung carcinogenesis, a groundbreaking study recently published in Oncotarget unveils a critical interaction between cigarette smoke exposure and impaired DNA repair mechanisms mediated by the Xeroderma Pigmentosum Group C (XPC) protein. This research deciphers how the combined assault of environmental toxins and molecular deficiency sets the stage for epithelial cell transformation, laying bare a “double hit” mechanism driving non-small cell lung cancer (NSCLC).
Lung cancer remains a leading cause of cancer-related deaths worldwide, with NSCLC accounting for approximately 85% of cases. The dual influence of carcinogen exposure, particularly from cigarette smoke, and genetic susceptibility has long been hypothesized. Yet, the molecular nexus linking environmental injury to DNA repair inefficiency had not been clearly delineated until now. The research team, led by Nawar Al Nasralla under the guidance of Catherine R. Sears, focused on the pivotal role of the Nucleotide Excision Repair (NER) protein XPC in maintaining genomic integrity against tobacco-induced damage.
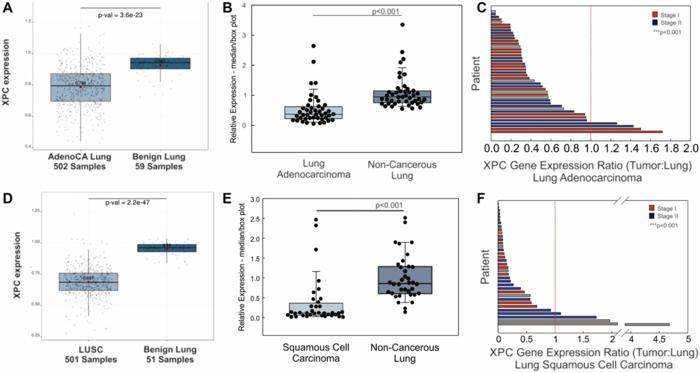
XPC serves as a critical DNA damage sensor within the global genome NER pathway. It identifies bulky DNA adducts and helix-distorting lesions frequently caused by polycyclic aromatic hydrocarbons and reactive oxygen species prevalent in cigarette smoke. Once damage recognition occurs, XPC recruits other repair proteins to excise and replace the aberrant DNA sequence, thus preventing mutagenesis. This study reveals that cigarette smoke significantly downregulates XPC mRNA expression in lung tissues, a finding corroborated by analyses of tumor samples from patients with lung adenocarcinoma and squamous cell carcinoma.
.adsslot_OlhaQkuADS{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_OlhaQkuADS{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_OlhaQkuADS{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The researchers utilized multiple data sources, including The Cancer Genome Atlas (TCGA) and frozen lung tissue specimens, to measure XPC expression levels. In both unmatched and patient-matched comparisons, malignant lung tissue exhibited marked reductions in XPC transcript abundance relative to adjacent benign lung. This consistent pattern of decreased DNA repair capacity suggests a compromised ability to cope with ongoing genotoxic stress in the pre-cancerous microenvironment.
Intriguingly, experimental exposure of normal human lung epithelial cells to cigarette smoke extract demonstrated exacerbated DNA damage accumulation and increased oxidative lesions, particularly when XPC expression was artificially suppressed. These findings illuminate a mechanistic basis for how diminished repair protein levels potentiate tobacco-related genotoxicity, escalating genomic instability and fostering malignant transformation. Conversely, established lung cancer cell lines manifested heightened resistance to smoke-induced damage despite low XPC, implying that tumor cells acquire alternative adaptive or repair pathways post-initiation.
This discovery underscores the concept of a “double hit” model in lung carcinogenesis whereby the first hit involves environmental exposure to mutagenic compounds in cigarette smoke, while the second hit entails an intrinsic deficiency in DNA repair enzyme function. Collectively, these hits synergize to overload the cellular DNA maintenance machinery, instigating irreversible mutations that drive epithelial cell dysplasia and neoplasia.
Importantly, this study illuminates the early events linking tobacco exposure and genetic vulnerability before cancer is clinically detectable. The pronounced susceptibility of normal lung cells lacking adequate XPC to cigarette smoke highlights a window of opportunity for intervention. Therapeutic strategies aimed at preserving or restoring XPC expression or function could potentially impede the progression from chronic injury to malignant disease.
Further, the differential responses observed between normal and cancerous cells to cigarette smoke-induced DNA damage hint at potential biomarkers for early lung cancer risk stratification. Assessing XPC mRNA levels in lung tissue or surrogate samples might provide a molecular signature of heightened cancer susceptibility, enabling targeted screening and personalized prevention.
The implications extend beyond lung cancer to other malignancies linked to environmental carcinogens where NER plays a protective role. By advancing our molecular understanding of how exogenous toxins impair endogenous repair systems, this research paves the way for innovative clinical applications, including pharmacologic enhancement of DNA repair pathways and refined risk assessment tools.
Moreover, this work prompts reconsideration of the cumulative effects of environmental and genetic factors in cancer biology. The abandonment of simplistic single-cause models in favor of integrated multidimensional frameworks can better capture the complexity of carcinogenesis and improve intervention outcomes.
In sum, the elucidation of XPC’s downregulation by cigarette smoke and its mechanistic consequences represents a milestone in lung cancer research. It validates the hypothesis that compromised NER capacity is a linchpin for tobacco-related epithelial carcinogenesis and identifies XPC as a strategic molecular target. As the authors conclude, enhancing DNA repair function may hold promise in mitigating lung cancer initiation among smokers and former smokers alike.
This study was supported by collaborative efforts from the Division of Pulmonary, Critical Care, Sleep, and Occupational Medicine in Indianapolis and the Richard L. Roudebush Veterans Affairs Medical Center. The authors declare no conflicts of interest, and the findings have broad translational potential warranting further exploration in clinical trials and biomarker development.
The research significantly bridges gaps in cancer molecular epidemiology, providing compelling evidence that DNA repair modulation is fundamental to cancer prevention strategies in high-risk populations exposed to tobacco carcinogens. Its novel insights set a framework for future investigations into prevention, early detection, and therapeutic innovation tailored to the molecular pathology of lung cancer.
Subject of Research:
Not applicable
Article Title:
Cigarette smoke and decreased DNA repair by Xeroderma Pigmentosum Group C use a double hit mechanism for epithelial cell lung carcinogenesis
News Publication Date:
20-May-2025
Web References:
http://dx.doi.org/10.18632/oncotarget.28724
Image Credits:
Copyright: © 2025 Nasrallah et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords:
cancer, DNA repair, DNA damage, lung adenocarcinoma, squamous cell carcinoma, Xeroderma Pigmentosum Group C (XPC)
Tags: cancer susceptibility factorscarcinogen interaction in lung cancercigarette smoke exposureDNA repair mechanismsenvironmental toxins and cancergenomic integrity and tobaccolung cancer researchmolecular deficiency in cancer developmentnon-small cell lung cancerNucleotide Excision Repair pathwaytobacco-induced DNA damageXeroderma Pigmentosum Group C