In the relentless pursuit to understand the complex mechanisms driving pancreatic cancer progression, a groundbreaking new study has unveiled the pivotal role of the tumor microenvironment’s acidity in promoting malignancy. Researchers have elucidated a novel molecular pathway implicating a specific long non-coding RNA (lncRNA), LOC100507424, as a key mediator in pancreatic cancer’s aggressive behavior under acidic conditions. This discovery not only advances the scientific comprehension of pancreatic cancer biology but also uncovers promising targets for future therapeutic intervention.
Pancreatic cancer remains one of the deadliest malignancies, notorious for its rapid progression, resistance to conventional treatments, and dismal prognosis. One of the critical challenges in combating this disease is its ability to adapt and thrive within the harsh microenvironment it creates. Tumor acidity, often a consequence of altered metabolism and insufficient blood supply, has emerged as a crucial factor in facilitating cancer cell survival, invasion, and metastasis. However, the molecular underpinnings that enable pancreatic cancer cells to exploit acidic niches within the tumor microenvironment have remained largely elusive—until now.
The new study delves deep into the role of lncRNAs, a class of regulatory RNA molecules that do not code for proteins but orchestrate gene expression through diverse mechanisms. LncRNAs have recently been recognized as vital players in cancer biology, influencing tumor initiation, progression, and response to therapy. Despite this growing awareness, their function in the context of acid-induced changes in pancreatic cancer remained poorly understood. Focusing on the lncRNA LOC100507424, previously associated with glioma stem cells, the researchers sought to decipher its contribution to pancreatic tumor aggression.
Clinical analyses revealed a significant upregulation of LOC100507424 in pancreatic cancer tissue samples compared to normal pancreatic tissues. Intriguingly, this upregulation was further amplified when pancreatic cancer cell lines were cultured under acidic conditions mimicking the tumor microenvironment. This observation suggested a direct link between the acidic milieu and lncRNA expression, hinting at an adaptive mechanism utilized by cancer cells to enhance their survival and invasiveness.
Experimental knockdown of LOC100507424 via targeted molecular techniques led to a marked reduction in pancreatic cancer cell proliferation, invasion, and metastatic potential in vitro. These functional assays established the lncRNA as more than a mere marker; it was a functional driver of malignant phenotypes. The inhibitory effects observed upon silencing LOC100507424 underscored its therapeutic relevance, positioning it as a potential biomolecular target for intervention.
Delving into the mechanistic aspect, the researchers uncovered that LOC100507424 exerts its pro-tumorigenic influence through transcriptional regulation mediated by the transcription factor E2F1, which in turn modulates the expression of FOXM1, a well-characterized oncogenic driver. FOXM1 is implicated in cell cycle progression, DNA damage response, and tumor metastasis across various cancers. The lncRNA’s interaction with E2F1 facilitates a chromatin environment conducive to FOXM1 transcription, thereby fueling the aggressive behavior of pancreatic cancer cells in acidic surroundings.
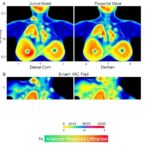
The study’s insights were further validated in vivo, where nude mice implanted with pancreatic cancer cells exhibiting high LOC100507424 expression developed significantly larger tumors compared to control groups. Conversely, silencing LOC100507424 attenuated tumor growth, highlighting the translational potential of these findings. The ability to manipulate this axis in living organisms provides a critical bridge between bench research and clinical applicability.
This research underscores the importance of considering the tumor microenvironment not merely as a passive backdrop but as an active participant shaping cancer progression through intricate molecular circuits. The acidic microenvironment, often a hallmark of solid tumors like pancreatic cancer, acts as a selective pressure that reprograms cancer cell behavior via non-coding RNA intermediaries. Such mechanisms reveal an added layer of complexity and present new avenues for therapeutic disruption.
Furthermore, targeting the lncRNA-LOC100507424/E2F1/FOXM1 axis bears significant potential for the development of targeted therapies that could overcome the innate resistance and adaptability of pancreatic cancer cells. Current treatment regimens are largely ineffective, reinforcing the urgent need for innovative strategies informed by molecular insights such as those presented here.
The identification of LOC100507424 as a critical nexus in pancreatic cancer progression also paves the way for its potential use as a biomarker. Its expression could serve as a prognostic indicator or as a measure to monitor therapeutic response, providing clinicians with valuable tools to personalize patient management.
It is worth noting that the study broadens our understanding of lncRNAs beyond their traditional conceptual roles, illustrating how these RNA molecules actively integrate environmental cues into the genetic regulatory networks that determine cell fate. This adds to a growing body of literature recognizing the functional versatility of lncRNAs in cancer and other pathologies.
Moreover, the elucidation of FOXM1 as a downstream effector consolidates previous reports of its oncogenic significance, now placed within the novel context of acidic microenvironment-driven regulation. Given FOXM1’s involvement in critical signaling pathways, inhibitors targeting this transcription factor might synergize with strategies aimed at lncRNA modulation to produce more effective therapeutic outcomes.
The study also highlights the dynamic interplay between epigenetic regulation and environmental factors within tumor ecosystems. Understanding how microenvironmental acidity influences chromatin remodeling and gene expression opens new frontiers in cancer biology, potentially applicable to other aggressive cancers exhibiting similar pathological traits.
In conclusion, the compelling evidence presented by Mu, Shi, Sun, and colleagues marks a substantial leap forward in delineating the molecular choreography underpinning pancreatic cancer progression. By uncovering the acidic microenvironment’s role in elevating lncRNA LOC100507424 and its consequent activation of the E2F1/FOXM1 axis, the research uncovers previously hidden vulnerabilities in one of the deadliest cancers. These insights lay a robust foundation for the development of novel diagnostic and therapeutic approaches that may ultimately transform pancreatic cancer care and improve patient outcomes worldwide.
As pancreatic cancer continues to pose monumental clinical challenges, studies like this illuminate the path toward more nuanced, mechanism-based treatments. The convergence of tumor microenvironment research and non-coding RNA biology heralds a new era in oncology where environment-informed molecular targeting could shift the tide in battling this formidable disease.
—
Subject of Research: Pancreatic cancer progression influenced by acidic tumor microenvironment and the role of lncRNA LOC100507424.
Article Title: The acidic microenvironment promotes pancreatic cancer progression via the lncRNA-LOC100507424/E2F1/FOXM1 axis.
Article References: Mu, D., Shi, Y., Sun, R. et al. The acidic microenvironment promotes pancreatic cancer progression via the lncRNA-LOC100507424/E2F1/FOXM1 axis. BMC Cancer 25, 655 (2025). https://doi.org/10.1186/s12885-025-14073-4
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14073-4
Tags: acidic tumor microenvironmentcancer cell survival mechanismscancer metastasis and tumor microenvironmentinvasive behavior of pancreatic tumorsLOC100507424 lncRNA rolelong non-coding RNA in cancermetabolic alterations in cancer cellsmolecular pathways in cancerpancreatic cancer progressionresistance to pancreatic cancer treatmentstherapeutic targets for pancreatic cancertumor acidity and malignancy