A new method that successfully designs serine hydrolase enzymes capable of catalyzing ester hydrolysis with high efficiency, demonstrates a computational approach for creating de novo enzymes that catalyze complex, multistep reactions.
The research was published in a paper titled, “Computational design of serine hydrolases,” in Science. The findings provide a framework for engineering enzymes with intricate active sites, expanding the possibilities for synthetic biocatalysts.
Designing functional enzymes from scratch remains a significant challenge in protein engineering. Traditional approaches often rely on inserting active sites into existing protein scaffolds, which can limit catalytic efficiency due to structural constraints. Until recently, computationally designed enzymes have had reduced efficiencies compared to natural enzymes, however, advances in machine learning and AI open new opportunities for more complex protein design options.

“We thought that designing a serine hydrolase, a well-characterized but fairly complex enzyme, would be an ideal model system to test out the latest AI tools, hopefully helping us uncover general methods that can be applied to the design of other enzymes,” co-lead author Anna Lauko, PhD, a postdoctoral researcher in the Baker lab at the University of Washington, shared with GEN.
Lauko and colleagues developed a novel machine learning model called PLACER (Protein-Ligand Atomistic Conformational Ensemble Reproduction), which predicts the active site conformations of designed enzymes.
“We can configure PLACER to predict the conformations of the active site in each step of the chemical reaction, allowing us to check computationally that the catalytic sidechains actually adopt the active conformation throughout the reaction mechanism,” Lauko explained to GEN.
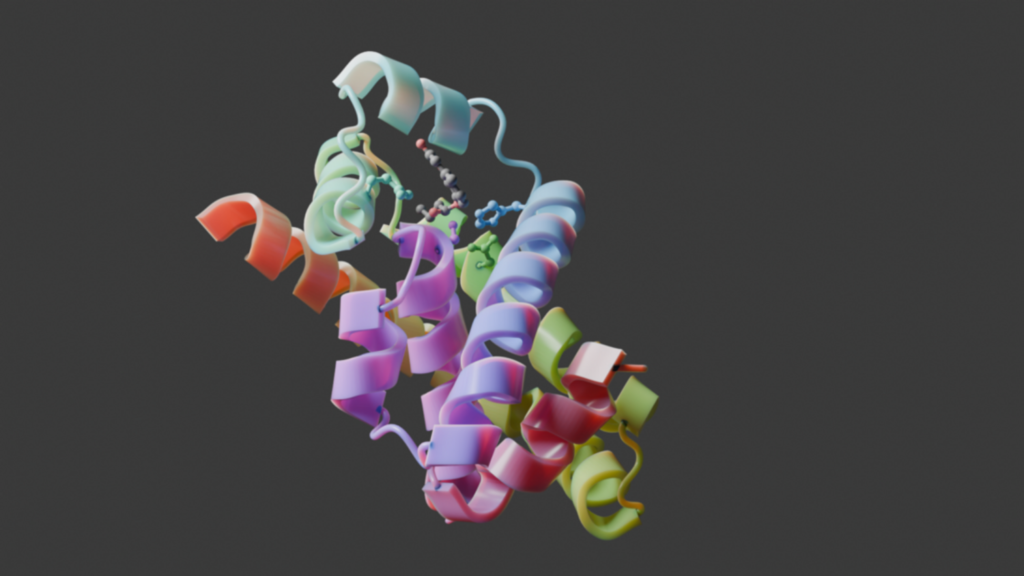
The team began their work using the established generative AI framework, RFdiffusion, to design proteins with complex catalytic sites. They then evaluated the active site conformation with PLACER, which makes predictions by analyzing the protein backbone, amino acid identities, and the chemical structures of bound molecules, allowing for a high degree of precision in enzyme design. With this method, the team successfully designed serine hydrolases that efficiently catalyze ester hydrolysis.
“Enzymes are very challenging to design because the conformation of the active site has to be atomically accurate for catalysis to occur at all,” co-lead author, Sam Pellock, PhD, also at the University of Washington, pointed out to GEN. “This requires precise design and prediction of not just the overall protein fold but also the conformations of individual sidechains, which is very challenging, especially for enzymes that contain multiple catalytic residues or use multi-step catalytic mechanisms.”
The approach eventually resulted in the team generating enzymes with minimal active site specification while maintaining high catalytic efficiency. Initially, the “designed enzymes could only perform the first half of the reaction mechanism before becoming inactivated,” Lauko reported to GEN.
The team redesigned simplified versions containing three of the five catalytic groups in the natural enzymes first, then pivoted to creating a more complex design containing all five catalytic groups found in the native serine hydrolases.
“We thought it might be easier to accurately design a simpler active site,” Lauko explained. “We tried making much more complex designs…and we were overjoyed when we saw that some of these designs could catalyze the whole reaction.”
Through experimental characterization of the created serine hydrolases, the team found that the novel enzymes retained folds unique from natural serine hydrolases with high catalytic efficiencies. These novel enzymes contained structures that closely matched the computational design models, as confirmed by crystal structure analysis. “We were really excited when we saw how well it matched our predicted structure,” Lauko shared.
By screening designed enzymes with active site preorganization, the team was able to preselect novel enzyme designs that had a higher likelihood of success in reality. The study’s findings highlight the potential of computational tools in enzyme engineering, particularly for creating biocatalysts with industrial and pharmaceutical applications. By integrating AI tools into their experimental methods, the researchers established an adaptable strategy for designing enzymes with tailored functions, with high utility in synthetic biology applications.
“We hope that the concepts and methods we used in this paper will be applicable to designing new enzymes in the future that act on important substrates or perform new chemistry,” Pellock concluded.