Scientists at University of California, San Diego (UCSD), have developed a new approach to destroying cancer stem cells (CSCs)—the hard-to-find cells that help cancers spread, come back after treatment, and resist therapy. The new approach uses artificial intelligence (AI) to identify treatments that can reprogram cancer stem cells, ultimately triggering them to self-destruct.
The machine learning-driven systems biology framework, called Cancer Associated Nodes for Differentiation Targeting, or CANDiT, only targets cancer cells, without affecting surrounding tissues. The researchers report on applying their approach to colon cancer, focusing on identifying targets that restore expression of CDX2. They suggest that their strategy could be a safer and more precise alternative to current therapeutic approaches.
“This isn’t just about colon cancer,” said Pradipta Ghosh, MD, professor of medicine and cellular & molecular medicine at UCSD School of Medicine. “CANDiT is an end-to-end human roadmap—we can apply it to any tumor, find the right targets, and finally take aim at the cells that have been the hardest to define, track, or treat.” Ghosh is senior author of the team’s published paper in Cell Reports Medicine, titled “CANDiT: A machine learning framework for differentiation therapy in colorectal cancer.”
Poor differentiation, marked by elevated stemness, is a hallmark of cancers, the authors wrote. And while differentiation therapy, which targets cancer stem cells to induce maturation, has shown success in hematologic malignancies, and this approach hasn’t yet been translated to carcinomas. “A major obstacle is the profound intra- and inter-tumoral heterogeneity that obscures identification of CSCs,” the team continued.
“Cancer stem cells are like shapeshifters,” said Ghosh. “They play hide-and-seek inside tumors. Just when you think you’ve spotted them, they disappear or change their identity. It’s like trying to hold on to a wet bar of soap in the shower.”
To outsmart these elusive cells, the team built a machine learning tool, CANDiT, that can identify new treatment targets for a specific tumor based on its unique genetics. The tool works by starting with a single key gene, one that healthy cells need to grow, but that is missing in aggressive cancers. From there, the tool identifies a network of genes related to the initial gene, suggesting treatment targets that can leverage this biochemical network to revert the cells to a healthier state.
For their reported study the team focused on identifying targets that restore expression of CDX2, a significant gene in colon cancer. Independent studies involving thousands of pateints have confirmed that “… CDX2-low CRCs are associated with worse overall survival (OS) and disease-free survival (DFS), independent of stage, mismatch repair status, or ethnicity,” the team noted. Absence of CDX2 in tumors is also associated with a number of adverse prognostic features, they reported, while CDX2 presence is associated with lower recurrence and mortality risk. “Collectively, these findings establish the restoration of CDX2 as a clinically meaningful and high-priority therapeutic goal.” However, they pointed out, “While its biological importance is widely recognized, no pharmacologic approach has yet succeeded in reliably inducing CDX2.”
For their study Ghosh and colleagues used CANDiT to rapidly scan the entire human genome in more than 4,600 unique human tumors, reflecting the genetic diversity typical of large, multi-center clinical trials. Their approach identified an unexpected new treatment target: a protein called PRKAB1, which helps cells respond to stress. By using an existing drug that activates this protein, the researchers were able to restore function of the CDX2 gene in colon cancer stem cells.
The drug, PF-06409577 (PF) is a clinical-grade PRKAB1 agonist, the team explained. “Although not yet approved for a clinical indication, PF has been deemed safe in a randomized, double-blind, placebo-controlled Phase I trial …” The researchers found that after treatment with the drug, the cancer stem cells began to behave more like normal healthy cells, but that wasn’t all.
“What surprised us most was that after we reprogrammed the cancer stem cells to behave like normal cells, they chose to self-destruct instead,” said first author Saptarshi Sinha, PhD, interim director of the Center for Precision Computational Systems Network (PreCSN), part of the Institute for Network Medicine (iNetMed) at UCSD School of Medicine. “It was as if they couldn’t live without their cancerous identity.”
![Pradipta Ghosh, M.D., professor at UC San Diego School of Medicine, director of HUMANOID, and senior author of the new study. [Kyle Dykes/UC San Diego Health Sciences]](https://www.genengnews.com/wp-content/uploads/2025/10/Low-Res_Ghosh-Pradipta-Portrait2-202504-300x200.jpg)
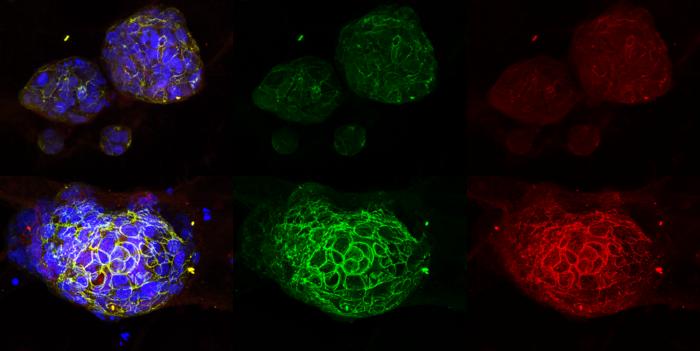
To demonstrate the clinical potential of this approach, the researchers were able to leverage UCSD’s HUMANOID™ Center, also part of (iNetMed), to successfully test the drug in patient-derived organoids (PDOs)—tiny, lab-grown replicas of human tumors. These organoids faithfully preserve the structure, behavior, and biology of real cancers, allowing researchers to safely and effectively test treatments in human tissues. Organoid experiments can streamline the process of bringing treatments to clinical trials, as many therapies that succeed in animal models ultimately fail in humans.
“It’s like doing clinical trials in a dish, which collapses timelines from years to months,” said Ghosh, who is also director of the HUMANOID Center. “We used a complete suite of cell analysis platforms at the Agilent Center of Excellence to measure not just whether a drug works, but how precisely and safely it works, before it ever reaches a patient.”
To explore the potential real-world impact of the treatment and identify who would benefit most from it, the researchers also identified a gene signature—a measurable pattern of gene activation—that can be used to predict how well someone might respond to this kind of therapy. Using advanced computer simulations of clinical trials, they tested this signature across 10 independent patient groups totaling more than 2,100 people, mirroring the diversity of large Phase III clinical trials. They found that using the drug to restore CDX2 in colon cancers could cut the risk of recurrence and death by up to 50%.
![This image shows the research team at HUMANOID, which creates patient-derived organoids to streamline the early drug discovery process. [Kyle Dykes/UC San Diego Health Sciences]](https://www.genengnews.com/wp-content/uploads/2025/10/Low-Res_HUMANOID-Team_Lab-705px-300x200.jpg)
Reporting on their collective results, the team stated, “Together, these findings identify a robust, target-derived 50-gene signature that not only captures the molecular response to CDX2-reinstatement but also stratifies survival risk. Therapeutic suppression of this signature emerges as a measurable and meaningful objective, with potential to improve patient outcomes.”
Sinha noted, “This was heartwarming, but not surprising. For decades, the Holy Grail of cancer has been its stem cells—resilient, elusive, and beyond our ability to identify or track them. They are able to outsmart every form of treatment, even the most advanced immunotherapies. To be able to track and selectively kill them brings us closer to rewriting the rules of cancer treatment.”
In their paper the team concluded, “Our demonstration that CDX2 serves as both a predictive biomarker and therapeutic effector for PF—a drug already deemed safe in humans (NCT02286882)—provides compelling justification for CDX2-guided clinical trials. More broadly, CANDiT opens the door to rational, scalable differentiation therapy across solid tumors.”
The researchers are now building on their momentum in collaboration with researchers across campus. This includes chemistry professor Jerry Yang, PhD, who has designed a more potent version of the compound with the goal of advancing it into clinical trials, and professor of surgery and UCSD Health surgical oncologist Michael Bouvet, MD, who is leading efforts to deploy CANDiT across multiple tumor types, including pancreatic, esophageal, gastric, biliary, and others. “It truly takes a village to get it right, and we’re fortunate to have the kind of partnerships that allow us to stay nimble yet impactful,” added Ghosh.
The team is also diving deeper into the question posed by their results: what made the cancer stem cells spontaneously die? Cracking that code could unlock an entirely new arsenal of therapies. Ghosh further stated, “By constantly anchoring small-scale organoid insights to Phase III–sized human diversity in the clinic, we can build discoveries that are rigorous, reproducible, and scalable, all without losing sight of the essentials of human disease. The potential of this approach to transform clinical medicine is not just immense—it’s inevitable.”