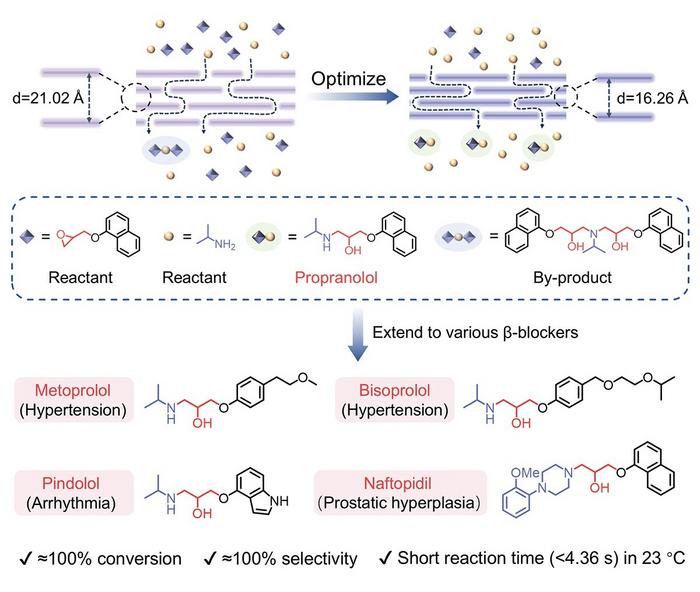
In a groundbreaking advancement poised to transform the pharmaceutical manufacturing landscape, a team of Chinese scientists has engineered an innovative membrane nanoreactor that dramatically enhances the synthesis efficiency and environmental sustainability of beta-blockers. This breakthrough centers on propranolol, a cornerstone medication widely prescribed for cardiovascular ailments including hypertension, arrhythmia, and angina, underscoring the direct clinical impact of this technological leap. The novel reactor not only expedites the synthetic process but also achieves near-perfect conversion and selectivity under remarkably mild conditions.
The research, spearheaded by Professor ZHANG Xiqi at the Technical Institute of Physics and Chemistry under the Chinese Academy of Sciences, reveals an amine-functionalized graphene oxide (NGO) membrane reactor that facilitates ultrafast, continuous-flow chemical synthesis. What sets this system apart is its ability to complete the conversion of key intermediates to propranolol with nearly 100% yield and selectivity in under 5 seconds at room temperature (23 °C). Such rapid reaction kinetics and high selectivity present a significant departure from conventional batch methods and heterogeneous catalysts that require elevated temperatures and prolonged reaction times.
Traditional synthesis of propranolol commonly relies on the ring-opening reaction of naphthyl glycidyl ether with isopropylamine. However, existing catalytic approaches have long struggled with several challenges including sluggish conversion rates, formation of unwanted side-products, and labor-intensive downstream purification processes. These obstacles have limited the scalability and economic viability of beta-blocker production. The newly developed NGO membrane reactor directly addresses these limitations by serving as a confined nanoscale reaction environment, which optimizes molecular interactions and reaction pathways.
.adsslot_ZLKy76IoN5{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_ZLKy76IoN5{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_ZLKy76IoN5{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
To construct these sophisticated membrane nanoreactors, the team employed vacuum-assisted filtration to assemble both acidic graphene oxide (GO) membranes and amine-functionalized graphene oxide (NGO) membranes. These membranes function as two-dimensional reactors where reactants diffuse through interlayer channels, providing a unique confined space that accelerates the ring-opening reaction. Remarkably, the NGO membrane outperformed its acidic GO counterpart by exhibiting a catalytic flux 4.36 times higher and a turnover frequency (TOF) about eight times greater, effectively demonstrating the superior catalytic properties imparted by amine functionalization.
Intriguingly, the researchers further refined the NGO membrane structure through carefully controlled mild thermal annealing to tune the interlayer spacing. As this spacing contracted, both the conversion efficiency and product selectivity were significantly enhanced. This tuning of interlayer distance was revealed through density functional theory simulations to reduce the activation energy required for propranolol formation, facilitating faster reaction rates. Simultaneously, the activation barrier for undesired byproducts increased, effectively suppressing their formation despite their inherent thermodynamic stability. This shift firmly establishes the reaction mechanism under kinetic control, dramatically improving the purity of the pharmaceutical product.
Another key innovation involved adjusting the reactant stoichiometry to mitigate the secondary reactions between residual naphthyl glycidyl ether and propranolol, which had previously led to byproducts that complicated purification processes. By increasing the molar ratio of isopropylamine to a 1:3 ratio relative to the glycidyl ether, the team attained near-complete conversion and exceptional product selectivity, illustrating the importance of precise reactant optimization within the membrane reactor environment.
When benchmarked against traditional catalytic systems, the NGO membrane reactor distinctly excelled with its ultra-short reaction times and operation under ambient temperatures, both of which are highly desirable from an energy efficiency and green chemistry perspective. Impressively, the turnover frequency of this membrane system reached 17.48 h⁻¹, substantially surpassing the 2.27 h⁻¹ achieved by NGO powder catalysts under identical experimental conditions. This leap in catalytic performance highlights the advantage of confining reactions within the two-dimensional nanospaces of the membrane.
Moreover, the versatility of this membrane reactor design was demonstrated by its successful application to the synthesis of other clinically relevant beta-blockers such as metoprolol, bisoprolol, pindolol, and naftopidil. This broader applicability signals a paradigm shift towards modular, high-throughput pharmaceutical manufacturing processes capable of adapting to diverse therapeutic targets without sacrificing efficiency or sustainability.
The implications of this research are profound for the pharmaceuticals industry, particularly in the context of increasing demand for more sustainable production methods. By combining ultrafast reaction kinetics, ambient operational conditions and precise molecular engineering within graphene oxide-based membranes, this innovation bridges the gap between laboratory-scale synthesis and industrial-scale continuous flow production.
This pioneering work was financially supported by significant national initiatives including the National Key R&D program of China, the Beijing Natural Science Foundation, and the National Natural Science Foundation of China, reflecting strong institutional commitment towards advancing green and efficient pharmaceutical technologies.
Publication of this study in the prestigious journal Matter on June 20, 2025, thus represents a landmark in catalysis and reaction engineering, offering new horizons for membrane-based nanoreactors as potent tools in medicinal chemistry and industrial manufacturing. The seamless integration of experimental design with computational modeling provides a robust foundation for future exploration of membrane nanoreactors across a spectrum of chemical transformations.
As the global healthcare industry continues to seek scalable, eco-friendly, and cost-effective drug synthesis methods, innovations such as the NGO membrane reactor developed by Professor ZHANG’s group point toward a sustainable era of pharmaceutical manufacturing, where efficiency, selectivity, and environmental responsibility converge.
Article Title: Membrane Nanoreactors for Mild and High-Efficiency Synthesis of β-Blockers
News Publication Date: 20-Jun-2025
Web References: https://doi.org/10.1016/j.matt.2025.102243
Image Credits: ZHANG’s Group
Keywords
Membrane biophysics; Membrane potentials; Biomaterials; Medical treatments; Cardiovascular disorders
Tags: advancements in pharmaceutical engineeringamine-functionalized graphene oxidebeta-blocker manufacturing innovationcardiovascular medication manufacturingchallenges in traditional drug synthesis methodscontinuous-flow chemical synthesisefficient pharmaceutical synthesisenvironmental sustainability in drug productionhigh-yield drug synthesis techniquesmembrane nanoreactor technologypropranolol synthesis breakthroughultrafast chemical reactions