Johnson & Johnson’s billion-dollar bet on a novel implant for heart failure hit a roadblock this week, with an FDA advisory panel unanimously voting against its risk-benefit profile.
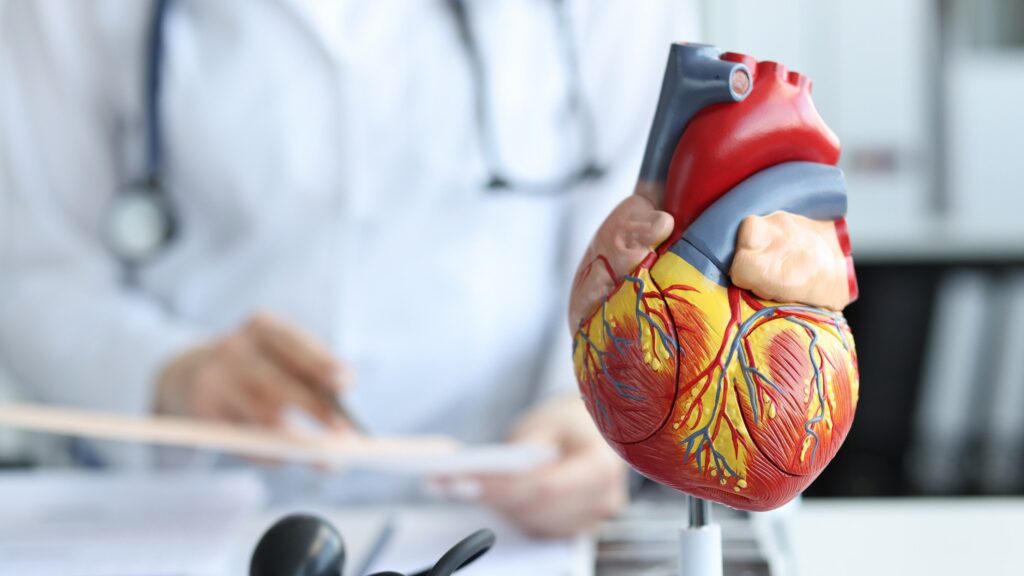
The V-Wave Ventura minimally invasive interatrial shunt aims to assist patients with severe disease and reduced ejection fraction, where the heart has trouble pushing out enough blood with each beat.
The permanent, hourglass-shaped device creates a small pathway between the left and right atria that evens out the pressure between the two chambers, with the goal of allowing the heart to pump more effectively and resolving symptoms such as difficulty breathing or excess fluid weighing down the lungs.
J&J inked a $1.7 billion deal to acquire V-Wave in 2024, including $600 million offered upfront, noting its device had the potential to be the first of its kind to reach the market. The implant previously received a breakthrough designation from the FDA in 2019.
Related
But the agency’s circulatory system devices panel appeared unconvinced this week, with some members calling for more clinical data.
The outside advisors analyzed the results of the Relieve-HF pivotal trial, which randomized 508 patients to either receive the shunt or serve as the control group. Out of those, 206 had heart failure with reduced ejection fraction, or HFrEF, and 302 had preserved ejection fraction, or HFpEF, split at a 40% cutoff.
The study showed the device was largely safe, with no major complications after implantation, but, after two years, there were no significant differences in the outcomes between the shunt and control groups—spanning a composite measurement of deaths, worsening cases of heart failure and hospitalizations, and changes in a symptom questionnaire.
At the same time, HFpEF patients who received the shunt ended up performing worse compared to the control group, while HFrEF patients logged some improvements.
At the FDA’s Dec. 3 panel meeting, members voted unanimously against whether the V-Wave Ventura shunt demonstrated effectiveness for its proposed heart failure indication and that its benefits did not outweigh its risks. On the question of whether there is reasonable assurance the implant is safe, nine members voted in favor and six voted against.
Related
“Given the number of other opportunities to change the natural history of heart failure and all its various phenotypes, I think the evidence bar is high,” said panel member Clyde Yancy, M.D., chief of the division of cardiology at Northwestern University Feinberg School of Medicine, in explaining his vote. “For everything we’ve done in the last decade or two to change outcomes for patients with heart failure, it’s always been driven by very high quality, indisputable evidence. That’s the standard that we should continue.”
Yancy added that development of the device should continue, given the needs of heart failure patients, and that any benefits should be more clearly established. “So my answer is no, but it preferably is, just not yet,” he said.
Related
“This procedure alters physiology in a tenuous population, and so I think more assurance of whether it is indeed effective and the basis for its effectiveness is really needed,” said David Yuh, M.D., a cardiothoracic surgeon at Brown University Health.
“Surgery is filled with examples of procedures embraced by desperate patients and physicians. Some forms of bariatric surgery, lung volume reduction surgery, even silicone injections, initially met with enthusiasm, have proven not to be durable in many cases,” Yuh said. “And I fear that this may fall in that category, but I think that remains to be seen.”
Christopher O’Connor, M.D., president of the Inova Schar Heart and Vascular Center, said Relieve-HF delivered “robust” data that set up the shunt’s continued development in trials with larger enrollment.
“As my previous colleagues have said, I’m highly encouraged by the depth of analysis that this group has done and they should be proud, and I hope that they will go forward with more studies,” he said.
Advisory committee votes are not the last word on an FDA green light, as the agency may decide to proceed as is or narrow the approved indication for a device.