In a groundbreaking new study poised to reshape our understanding of auditory health and aging, researchers have uncovered a pivotal role for fibroblast growth factor 13 (FGF13) in safeguarding the delicate neural architecture of the inner ear. As millions worldwide grapple with the progressive decline in hearing acuity associated with aging, this discovery offers a beacon of hope, promising new avenues for therapeutic intervention aimed at preventing or mitigating age-related hearing loss (ARHL).
Hearing loss that emerges gradually over time, known medically as presbycusis, results primarily from cumulative damage to the sensory and neural elements within the cochlea. Central to this process is the degeneration of spiral ganglion neurons (SGNs) and the ribbon synapses that bridge these neurons with inner hair cells. These specialized synapses are essential for the rapid and precise transmission of auditory signals to the brain, enabling the nuanced perception of sound. The gradual attrition of these synaptic structures underlies the diminished hearing sensitivity and speech discrimination difficulties hallmarking ARHL.
The study led by Yin, Cao, Yang, and their colleagues takes a molecular lens to this problem, exploring how FGF13, a member of the fibroblast growth factor family known for its roles in neural development and cellular protection, influences cochlear integrity in aging models. Their findings, published in the latest volume of Cell Death Discovery, illuminate how FGF13 functions as a neuroprotective agent within the cochlea, shielding SGNs and ribbon synapses from inflammatory and oxidative insults that accumulate with advancing age.
.adsslot_4zLepOH6T3{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_4zLepOH6T3{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_4zLepOH6T3{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
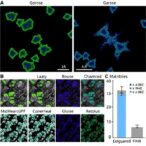
Detailed histological analyses reveal that FGF13 expression is markedly diminished in the cochleae of aged subjects, correlating strongly with increased degeneration of spiral ganglion neurons and synaptic structures. The research team employed both genetically modified mouse models and in vitro assays to determine that restoring or augmenting FGF13 levels significantly attenuates neuronal loss and synaptic disassembly. This neuroprotection translates into improved auditory brainstem responses, a key measure of hearing function, underscoring the functional relevance of FGF13 within the auditory system.
At the cellular level, FGF13 appears to exert its protective effects by modulating intracellular signaling pathways that control neuronal survival and synaptic stability. Notably, the activation of anti-apoptotic cascades and the attenuation of pro-inflammatory kinase activity form the biochemical backbone of FGF13’s mechanism. The protein’s ability to foster cytoskeletal integrity within spiral ganglion neurons may also stabilize the cytoplasmic scaffolding crucial for synaptic maintenance, as ribbon synapses rely heavily on precise cytoskeletal arrangements for their ultra-rapid vesicle cycling.
This intricate interplay between FGF13 and neuronal resilience is significant in that it identifies molecular targets previously unexplored in the context of hearing preservation. Unlike traditional approaches that focus largely on hair cell regeneration or cochlear implants, targeting synaptic and neuronal health addresses the root cause of signal transmission failure. This is particularly vital given that neuronal death is often irreversible, and synaptic deficits precede or accompany sensory cell loss during ARHL progression.
The implications of this research extend beyond the realm of auditory neuroscience. By elucidating how FGF13 safeguards ribbon synapses—unique presynaptic structures characterized by electron-dense ribbons that tether synaptic vesicles—this study contributes crucial insight into synaptopathy, a condition increasingly recognized as a common denominator in various neurodegenerative diseases. Understanding the molecular framework preserving synapse integrity could inform strategies for other sensory or central nervous system disorders featuring synaptic loss.
Moreover, the study lays the groundwork for potential pharmacological interventions aimed at modulating FGF13 activity or expression. The researchers show that gene therapy approaches to boost FGF13 in aged cochleae yield promising results in preclinical models, enhancing not only synaptic preservation but also auditory thresholds. This opens exciting prospects for the development of targeted treatments that could halt or slow the progression of hearing impairment in aging populations.
Of equal importance is the study’s methodological rigor, combining molecular biology, electrophysiology, and advanced imaging techniques such as confocal microscopy with immunolabeling of synaptic proteins. This multidisciplinary approach allowed for the precise mapping of FGF13’s influence on both macrostructural cochlear anatomy and microstructural synapse morphology. Additionally, the use of quantitative PCR and Western blotting confirmed the dynamic regulation of FGF13 expression associated with cellular stress responses.
Age-related hearing loss remains a significant public health challenge, with far-reaching consequences including social isolation, cognitive decline, and reduced quality of life. The identification of FGF13 as a natural defender against the neural degeneration underlying ARHL brings scientists one step closer to effective interventions. Unlike previous treatments that have been largely palliative, the possibility of molecular therapies that proactively preserve auditory synapses represents a paradigm shift.
Furthermore, the study prompts a reevaluation of the pathophysiological cascade leading to hearing loss, suggesting that maintaining synaptic health is as crucial as protecting hair cells themselves. Future research inspired by these findings may explore combinatorial therapies that target both cellular populations, optimizing auditory system resilience throughout the lifespan.
This breakthrough also invites exploration into the regulation of FGF13 under normal and pathological conditions. Understanding the upstream factors that control its expression and activity could reveal modifiable environmental or lifestyle influences capable of enhancing natural protective mechanisms in auditory neurons. Additionally, the role of FGF13 in other forms of hearing impairment, such as noise-induced or ototoxic drug-related hearing loss, warrants investigation.
The discovery that a single growth factor protein such as FGF13 can exert profound neuroprotective effects underscores the complexity and precision of the inner ear’s molecular environment. It also exemplifies the importance of basic scientific inquiry into cell signaling pathways as foundations for translational medicine. As researchers continue to unravel the molecular underpinnings of sensory aging, the prospect of restoring youthful hearing function becomes increasingly tangible.
In conclusion, the work by Yin, Cao, Yang, and colleagues represents a significant stride toward combating age-related auditory decline. By highlighting FGF13’s critical role in protecting spiral ganglion neurons and ribbon synapses, the study paves the way for innovative treatments that could preserve hearing health into advanced age. This landmark research not only enhances our understanding of cochlear biology but also ignites hope for millions affected by hearing loss worldwide.
Subject of Research: Prevention of age-related hearing loss through neuroprotection of spiral ganglion neurons and ribbon synapses by FGF13
Article Title: FGF13 prevents age-related hearing loss by protecting spiral ganglion neurons and ribbon synapses from injury
Article References:
Yin, H., Cao, H., Yang, J. et al. FGF13 prevents age-related hearing loss by protecting spiral ganglion neurons and ribbon synapses from injury. Cell Death Discov. 11, 307 (2025). https://doi.org/10.1038/s41420-025-02607-5
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41420-025-02607-5
Tags: age-related hearing loss preventionauditory signal transmissioncochlea sensory elementsFGF13 role in auditory healthfibroblast growth factor familyinner ear neural architectureneuroprotection in agingpresbycusis and neural degenerationresearch on hearing acuity declinespiral ganglion neurons protectionsynaptic structures in hearingtherapeutic interventions for hearing loss