In the ever-evolving landscape of chemical synthesis, the demand for innovative and environmentally benign methods is growing exponentially. Fluorination, a pivotal process responsible for incorporating fluorine atoms into organic compounds, plays a critical role across a broad spectrum of industries, including pharmaceuticals, agrochemicals, and advanced materials design. Despite the immense utility of fluorine-containing compounds, the chemical community faces significant challenges in developing fluorinating agents that combine efficacy, safety, and sustainability. Traditional reagents often suffer from issues such as poor solubility, high hygroscopicity, or hazardous handling conditions, creating substantial barriers for widespread industrial implementation.
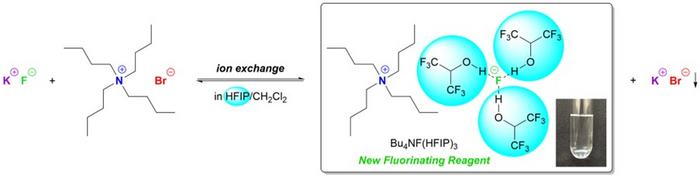
Addressing these challenges, a pioneering research team at the Shibaura Institute of Technology, led by Professor Toshiki Tajima, has introduced a groundbreaking approach that marries simplicity and green chemistry principles. Their work culminated in the synthesis of a novel quaternary ammonium-based fluorinating reagent, R4NF(HFIP)3, formed through an ion-exchange reaction between potassium fluoride (KF) and tetrabutylammonium bromide (Bu4NBr) in the presence of hexafluoroisopropanol (HFIP). This new complex boasts remarkable properties, such as drastically reduced hygroscopicity and enhanced solubility, overcoming the classic limitations of its parent compounds.
One of the fundamental hurdles with potassium fluoride has been its notoriously low solubility in organic solvents, which restricts its utility despite its low cost and relative safety. Meanwhile, quaternary ammonium fluorides like Bu4NF, though more reactive, pose practical problems due to their high hygroscopic nature, necessitating careful storage and handling. Professor Tajima’s insight into leveraging HFIP as a coordinating solvent allowed for a novel pathway where KF could be solubilized efficiently, producing a stable and easily manageable reagent. This represents a significant stride in the realm of fluorine chemistry toward greener alternatives.
.adsslot_a5JWM2CpnQ{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_a5JWM2CpnQ{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_a5JWM2CpnQ{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The synthesis process commences with separate dissolution stages: KF is introduced to HFIP, while Bu4NBr is dissolved in dichloromethane. When these two solutions are combined and stirred at ambient conditions for 30 minutes, an ion exchange ensues, yielding the tri(HFIP)-coordinated fluorinating complex Bu4NF(HFIP)3. The product is a viscous, clear liquid that demonstrated a consistent composition as confirmed by nuclear magnetic resonance (NMR) spectroscopy. Such straightforward preparation not only reduces synthetic complexity but also democratizes access to the reagent across various scales of research and industrial production.
A critical advantage of the newly synthesized reagent is its drastically lowered hygroscopicity compared to Bu4NF. This property significantly extends the shelf life and ease of handling, making the complex an attractive candidate for commercial adoption. Furthermore, the method’s foundational principle—utilizing a simple ion exchange reaction with readily available and inexpensive reagents—aligns perfectly with the tenets of sustainable chemistry. By minimizing the need for complicated purification protocols and hazardous solvents, this innovation marks a meaningful step forward in reducing the environmental footprint of fluorination processes.
Moreover, beyond the immediate success with Bu4NBr, the researchers successfully extended this synthetic strategy to other quaternary ammonium bromides, thereby broadening the scope of accessible fluorinating agents with tailored properties. This versatility underscores the potential of the tri(HFIP) coordination motif as a modular approach for designing and optimizing fluorination reagents, paving the way for customized applications in diverse chemical settings.
Electrochemical fluorination (ECF), an area where fluorinating reagents must exhibit not only reactivity but also stability under electrochemical conditions, stands to benefit enormously from this development. The Bu4NF(HFIP)3 complex was demonstrated to be highly effective in ECF processes, enabling the clean and selective introduction of fluorine atoms under mild conditions. This could revolutionize methodologies in organic electrosynthesis, enabling safer and more sustainable routes to critical fluorinated products.
Professor Tajima highlights the broader implications of this work, emphasizing that the newly devised reagent holds promise for numerous applications, ranging from pharmaceutical intermediates to molecular probes used in positron emission tomography (PET). The ease of synthesis, coupled with enhanced reagent stability and performance, could transform current practices, facilitating quicker development cycles and lowering costs associated with fluorination-based modifications.
The discovery also resonates with ongoing global efforts to reduce hazardous chemical waste and environmental contamination stemming from industrial chemical processes. Fluorination reactions traditionally involve aggressive reagents and conditions, but innovations like Bu4NF(HFIP)3 open doors to cleaner, safer alternatives that reconcile industrial demand with environmental stewardship. Such advances are critical as regulatory landscapes tighten and as industries increasingly prioritize green manufacturing paradigms.
It is worth noting that the publication of these findings, made publicly available in Chemical Communications, details not only the synthetic routes but also comprehensive characterization data corroborating the properties and efficacy of the new reagent. The study exemplifies a thoughtful integration of organic electrochemistry principles with practical synthetic chemistry, delivering both mechanistic insights and applicable technologies.
The success of this research is firmly rooted in the academic rigor and interdisciplinary approach championed by the Shibaura Institute of Technology, an institution recognized for its commitment to engineering solutions that harmonize with societal and environmental needs. By focusing on “learning through practice,” the institute fosters innovation that transcends laboratories and impacts real-world chemical challenges, embodied by Professor Tajima’s group accomplishment.
In summary, the facile synthesis of R4NF(HFIP)3 complexes signifies a landmark achievement in the pursuit of greener fluorination methodologies. This development not only resolves enduring technical limitations but also charts a sustainable path forward for the synthesis of fluorine-containing organic compounds. The reagent’s stability, ease of manufacture, and enhanced reactivity are poised to stimulate research activity and facilitate industrial adoption, rendering it a transformative tool in the chemist’s repertoire.
This breakthrough, published on May 25, 2025, in Chemical Communications, is expected to catalyze further innovation in green chemistry, electrochemical synthesis, and the broader chemical manufacturing sector. As fluorination continues to underpin critical advances in materials science, medicine, and energy, the introduction of such safer and more effective reagents represents a pivotal moment in expanding the boundaries of sustainable chemical synthesis.
Subject of Research:
Not applicable
Article Title:
Facile synthesis of R4NF(HFIP)3 complexes from KF and their application to electrochemical fluorination
News Publication Date:
25-May-2025
Web References:
https://doi.org/10.1039/D5CC01341K
https://www.shibaura-it.ac.jp/en/
References:
DOI: 10.1039/d5cc01341k
Image Credits:
Professor Toshiki Tajima from Shibaura Institute of Technology, Japan
Keywords
Green chemistry, Fluorination, Electrochemical reactions, Electrochemistry, Environmental chemistry, Chemical engineering, Nanotechnology, Energy, Materials science, Sustainable development
Tags: agrochemical and pharmaceutical applicationsenvironmentally friendly fluorinating agentsfluorination process advancementsgreen chemistry innovationshexafluoroisopropanol applicationsindustrial implementation of fluorine compoundsnovel synthetic approaches in organic chemistryovercoming solubility challenges in chemistrypotassium fluoride alternativesquaternary ammonium fluorinating reagentssafe handling of fluorine reagentssustainable chemical synthesis methods