A groundbreaking review recently published in Genes & Diseases shines a spotlight on the intricate and critical process of ribosome biogenesis and its profound implications for liver health and disease. Ribosomes, often hailed as the cellular factories for protein synthesis, are not only fundamental for normal liver function but also play pivotal roles in liver regeneration, viral infections such as hepatitis C virus (HCV), metabolic disorders including nonalcoholic fatty liver disease (NAFLD), and a spectrum of chronic liver diseases culminating in liver cancer. This comprehensive analysis deciphers the molecular interplay between ribosome assembly and liver pathology, revealing new avenues for targeted therapies.
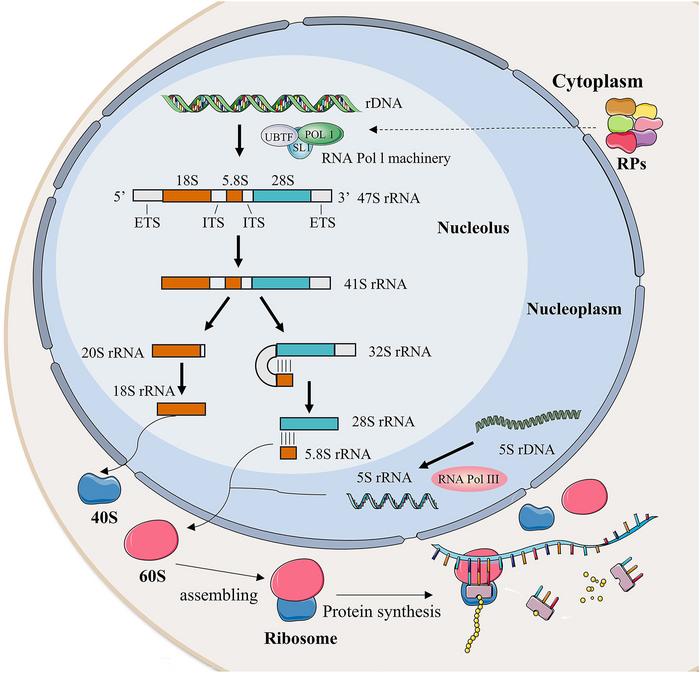
Ribosome biogenesis is a multifaceted and highly orchestrated process that starts in the nucleolus, a specialized subnuclear structure, where ribosomal RNA (rRNA) is transcribed primarily by RNA polymerase I. The process entails the synthesis and processing of precursor rRNA transcripts, coordinated incorporation of ribosomal proteins, and assistance from a large cohort of assembly factors. Once these components are assembled into pre-ribosomal subunits, they are exported to the cytoplasm for maturation into functional ribosomes. This entire biogenetic pathway ensures that the cell maintains a robust protein production capacity indispensable for liver cells, which must frequently regenerate and respond to metabolic demands.
The liver’s phenomenal capacity to regenerate after injury is intimately tied to ribosome biogenesis. Hepatocytes ramp up protein synthesis machinery to facilitate replication and repair, a process heavily reliant on efficient ribosome production. Any disruption in ribosome assembly impairs this regenerative capability, predisposing liver tissue to damage and functional decline. This mechanistic insight underscores why ribosome biogenesis is central not only to normal liver physiology but also to the pathology of liver diseases triggered by injury or infection.
.adsslot_CXPeprMHgQ{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_CXPeprMHgQ{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_CXPeprMHgQ{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
In the context of hepatitis C virus (HCV) infection, ribosomes assume an additional, more sinister role. HCV exploits the host’s translational machinery for viral protein synthesis and replication. The viral lifecycle is tightly dependent on the host’s ribosomal function, rendering ribosome biogenesis a double-edged sword in infection scenarios. Notably, therapeutic strategies targeting components of ribosome synthesis or function emerge as promising candidates to hinder viral propagation, representing a novel front in antiviral drug development.
Nonalcoholic fatty liver disease (NAFLD), a metabolic disorder characterized by excessive fat accumulation within hepatocytes, has also been linked to the modulation of ribosomal activity. Enhanced ribosome biogenesis correlates with increased lipogenesis, the process by which fatty acids and triglycerides are synthesized. This suggests that ribosomes are actively involved in metabolic reprogramming that underlies NAFLD progression. By targeting ribosomal pathways, there may be potential to modulate metabolic derangements and attenuate disease severity.
Chronic liver diseases such as fibrosis and cirrhosis develop following persistent injuries that promote excessive extracellular matrix production and scarring. At the molecular level, activated hepatic stellate cells (HSCs) drive this fibrogenic response, and this activation is closely linked to aberrant ribosome biogenesis. Overactive ribosomal machinery in HSCs can fuel their proliferation and secretion of matrix components, exacerbating fibrosis. A deeper understanding of ribosome assembly within these cells offers prospects for interrupting the fibrotic cascade at its source.
Hepatocellular carcinoma (HCC), the most common form of liver cancer, stands out as a disease profoundly influenced by dysregulated ribosome biogenesis. Tumor cells frequently exhibit elevated ribosome production to meet their increased protein synthesis demands, enabling rapid growth and proliferation. Elevated rRNA transcription, ribosomal protein overexpression, and alterations in assembly factors collectively contribute to oncogenesis. Importantly, this overdrive in ribosome biogenesis represents a vulnerability that can be exploited therapeutically.
Pharmacological inhibitors designed to disrupt ribosome biogenesis are in various stages of development and testing. One notable compound, CX-5461, targets RNA polymerase I-mediated rRNA transcription, effectively dampening ribosome production. Preclinical studies demonstrate that CX-5461 can induce nucleolar stress and apoptosis selectively in cancer cells, highlighting its potential as a liver cancer therapeutic. These agents exemplify a shift toward targeting the tumor’s protein synthesis platform rather than its genetic mutations alone.
Beyond monotherapy, there is optimism surrounding the combination of ribosome-targeting drugs with standard chemotherapy regimens. Such combinations could enhance treatment efficacy by simultaneously crippling cancer cells’ protein production capability and conventional cytotoxic pathways. This multifaceted attack might overcome therapeutic resistance often encountered in advanced liver cancers, improving patient outcomes and survival rates.
The molecular pathways linking ribosome biogenesis to liver pathology are complex, involving numerous signaling networks and checkpoints. These include regulatory feedback loops ensuring cellular homeostasis and stress responses that either augment or suppress ribosome assembly under pathological conditions. Elucidating these pathways furnishes a blueprint for designing highly specific interventions that minimize off-target effects and toxicity.
The implications of this review extend beyond liver diseases, inviting a broader research interest into ribosome biogenesis as a central hub in multiple disease states. The approach of manipulating the cell’s translational apparatus challenges traditional paradigms and exemplifies precision medicine at a subcellular level. By honing in on the molecular underpinnings of ribosome production, scientists are pioneering novel, targeted therapies that promise to revolutionize how we treat liver ailments.
In conclusion, ribosome biogenesis stands at the crossroads of liver health and disease, from regeneration following injury to the pathophysiology of viral infections, metabolic disorders, fibrosis, and cancer. This review underscores not only the biological significance of ribosome assembly but also its therapeutic potential, marking it as a vital focus in hepatology research. Harnessing our understanding of this process could usher in a new era of effective, targeted treatment strategies for some of the most challenging liver diseases.
Subject of Research: Ribosome biogenesis and its role in liver diseases
Article Title: Ribosome biogenesis: A central player in liver diseases
News Publication Date: 2025
References: Wei Luo, Jing Zhou, Yongmin Yan, Xuezhong Xu, Ribosome biogenesis: A central player in liver diseases, Genes & Diseases, Volume 12, Issue 5, 2025, 101512
Image Credits: Genes & Diseases
Keywords: Cancer genetics, Ribosome biogenesis, Liver regeneration, Hepatitis C virus, Nonalcoholic fatty liver disease, Liver fibrosis, Cirrhosis, Hepatocellular carcinoma, CX-5461, RNA polymerase I inhibitors
Tags: assembly factors in ribosome biogenesischronic liver disease treatment strategieshepatitis C virus and ribosome assemblyliver regeneration and ribosomesmetabolic disorders and liver healthmolecular pathways in liver pathologynonalcoholic fatty liver disease mechanismsnucleolus and ribosome productionprotein synthesis in liver functionribosome biogenesis in liver diseaserole of ribosomal RNA in liver diseasetargeted therapies for liver cancer