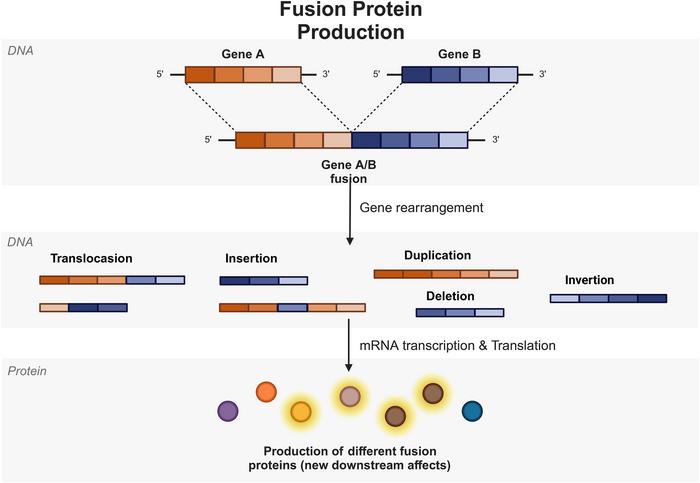
In the relentless quest to unravel the complexities of cancer biology, fusion genes have emerged as critical molecular culprits driving oncogenesis. These hybrid genes arise from intricate chromosomal rearrangements such as translocations, insertions, deletions, duplications, and inversions. By fusing segments of two originally separate genes, these rearrangements generate novel chimeric proteins whose altered functionalities can profoundly disrupt cellular homeostasis and ignite malignant transformation. Recent comprehensive studies have underscored the pervasive influence of fusion genes across a panoply of cancer types, ranging from hematological malignancies to solid tumors including lung, thyroid, and prostate cancers, heralding a new era in precision oncology.
At the molecular level, fusion genes often encode aberrant fusion proteins with unique domain architectures that co-opt signaling pathways critical for cell growth, survival, and differentiation. Unlike normal proteins, these chimeric entities can constitutively activate oncogenic cascades, dismantle tumor suppressor functions, and reprogram cellular metabolism. For instance, the BCR-ABL fusion protein, archetypal in chronic myeloid leukemia, combines the breakpoint cluster region (BCR) with the Abelson tyrosine kinase (ABL), resulting in constitutive tyrosine kinase activity that drives unchecked proliferation. Similarly, fusion proteins like EML4-ALK in non-small cell lung cancer and PML-RARα in acute promyelocytic leukemia catalyze oncogenesis via distinct yet potent mechanisms, exemplifying the functional diversity and tissue specificity of fusion oncogenes.
The discovery and characterization of these fusion genes have revolutionized cancer diagnostics. Advanced genomic technologies, including next-generation sequencing (NGS) and RNA sequencing (RNA-seq), now enable the precise identification of fusion transcripts with remarkable sensitivity. This molecular precision facilitates the stratification of tumors beyond histopathological criteria, permitting clinicians to delineate subtypes with differential prognoses and therapeutic vulnerabilities. Moreover, the advent of liquid biopsy techniques empowers real-time monitoring of fusion gene burden in circulating tumor DNA or RNA, providing minimally invasive windows into tumor evolution and therapeutic response dynamics.
.adsslot_Vj5eOtoAbR{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_Vj5eOtoAbR{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_Vj5eOtoAbR{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Therapeutically, fusion genes represent a golden target. The development of targeted inhibitors against fusion proteins, such as tyrosine kinase inhibitors (TKIs) targeting BCR-ABL or ALK fusions, have dramatically altered clinical outcomes in affected patient populations. These agents afford enhanced efficacy with reduced systemic toxicity compared to conventional cytotoxic chemotherapies. However, the clinical journey is not without challenges. Resistance mechanisms mediated by secondary mutations, activation of compensatory pathways, or phenotypic plasticity pose formidable obstacles that necessitate continual drug development and combination strategies to sustain remission and prevent relapse.
A critical frontier remains in understanding the functional heterogeneity of fusion genes and their contextual interactions within the tumor microenvironment. This complexity influences cancer aggressiveness, immune evasion, and metastatic propensity. Emerging research is elucidating how fusion proteins modulate cell-cell communication, extracellular matrix remodeling, and immune checkpoint landscapes, underscoring the multifaceted roles these genetic alterations play beyond autonomous tumor cell proliferation. Deciphering these intricate networks will be pivotal for bespoke interventions in resistant or refractory cancers.
Notably, the biogenesis of fusion genes involves a spectrum of genomic instability processes. DNA double-strand breaks, erroneous repair via non-homologous end joining (NHEJ), and replication stress contribute to chromosomal rearrangements that generate fusion transcripts. The landscape of fusion gene formation is further complicated by the presence of complex structural variants and cryptic rearrangements often elusive to routine cytogenetic analysis. Enhanced bioinformatic pipelines and integrative omics approaches are thus essential to capturing the full spectrum of fusion events and their oncogenic consequences.
Immense promise lies in the deployment of next-generation inhibitors tailored to specific fusion-driven malignancies. Structural biology and high-throughput screening are expediting the discovery of agents capable of targeting unique fusion protein interfaces or downstream effectors. Additionally, combinatorial therapeutic regimens that integrate fusion protein inhibition with immunotherapy, epigenetic modulators, or metabolic interventions hold significant potential in overcoming resistance and achieving durable remissions.
The clinical translation of fusion gene research also benefits from refined diagnostic assays designed for rapid detection and quantification. Multiplexed PCR assays, fluorescence in situ hybridization (FISH), and emerging CRISPR-based diagnostics enhance the clinical laboratory toolkit, enabling timely therapeutic decision-making. Coupling these technologies with artificial intelligence-driven predictive models further augments the precision medicine paradigm, optimizing individual patient management.
Importantly, the implications of fusion genes extend beyond oncology. Their roles in developmental disorders, congenital abnormalities, and other disease states are subjects of active investigation, suggesting a broader biological significance. Understanding fusion gene biology may reveal novel pathogenic mechanisms and therapeutic targets across diverse medical disciplines.
Despite remarkable advancements, the field confronts unresolved challenges. Heterogeneity within and between tumor types, the temporal dynamics of fusion gene expression during disease progression, and the interplay with host genomic and epigenomic landscapes require elucidation. Addressing these gaps through multidisciplinary collaboration and innovative research methodologies will accelerate the realization of fusion gene-targeted therapies as standard of care.
In summation, fusion genes stand as linchpins in the molecular architecture of many cancers, offering both profound insights into tumorigenesis and actionable therapeutic targets. The fusion of cutting-edge genomic technologies with drug innovation portends a transformative impact on cancer care, aligning with the overarching objectives of precision medicine. As the molecular taxonomy of cancers is refined, fusion genes will undoubtedly remain at the vanguard of translational oncology, offering renewed hope to patients worldwide.
Subject of Research: Fusion genes in cancer
Article Title: Fusion genes in cancers: Biogenesis, functions, and therapeutic implications
News Publication Date: 2025
References: Haiqiong Tang, Qiu Peng, Linda Oyang, Shiming Tan, Xianjie Jiang, Zongyao Ren, Xuemeng Xu, Mengzhou Shen, Haofan Li, Mingjing Peng, Longzheng Xia, Wenjuan Yang, Shizhen Li, Jiewen Wang, Yaqian Han, Nayiyuan Wu, Yanyan Tang, Jinguan Lin, Qianjin Liao, Yujuan Zhou, Fusion genes in cancers: Biogenesis, functions, and therapeutic implications, Genes & Diseases, Volume 12, Issue 5, 2025, 101536
Image Credits: Genes & Diseases
Keywords: Cancer genetics, Fusion genes, Oncogenes, Precision medicine, Targeted therapy
Tags: BCR-ABL fusion in leukemiachromosomal rearrangements in cancerEML4-ALK in lung cancerfusion genes in cancerfusion proteins and signaling pathwayshematological malignancies and fusion genesimpact of chimeric proteins on cancermolecular mechanisms of oncogenesisPML-RARα and acute promyelocytic leukemiaprecision oncology and fusion genessolid tumors and fusion gene influencetargeted therapies for fusion gene-driven cancers