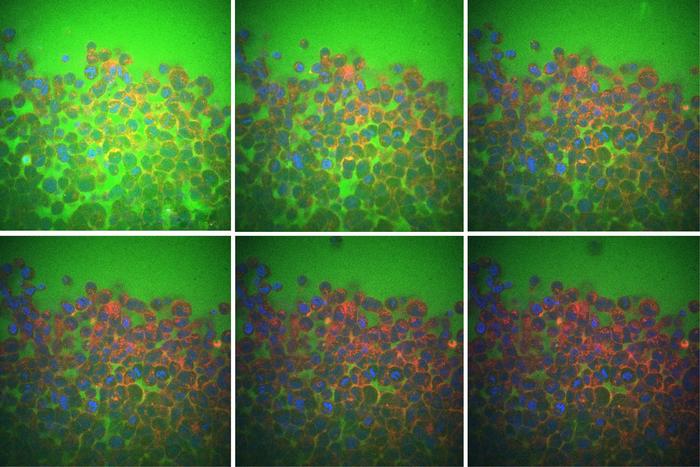
These images use color markers—blue for nuclei, red for cell membranes, and green for fluid—to show that spaces between cells shrink as fluid moves out during tissue compression, from left to right and top to bottom. [Courtesy of Ming Guo, Fan Liu, et al]
Water makes up around 60% of the human body. More than half of this water is inside the cells that make up organs and tissues, and much of the remaining water flows in the spaces between cells.
MIT engineers have found that this intercellular fluid plays a major role in how tissues respond when squeezed, pressed, or physically deformed. The researchers showed that when a tissue is pressed or squeezed, it is more compliant and relaxes more quickly when the fluid between its cells flows easily. When the cells are packed together and there is less room for intercellular flow, the tissue as a whole is stiffer and resists being pressed or squeezed. The findings could help scientists understand how cells, tissues, and organs physically adapt to conditions such as aging, cancer, diabetes, and certain neuromuscular diseases.
The results challenge conventional wisdom, which has assumed that a tissue’s compliance depends mainly on what’s inside, rather than around, a cell. Now that the researchers have shown that intercellular flow determines how tissues will adapt to physical forces, these new insights can be applied to understand a wide range of physiological conditions, including how muscles withstand exercise and recover from injury, and how a tissue’s physical adaptability may affect the progression of aging, cancer, and other medical conditions.
“People know there is a lot of fluid between cells in tissues, but how important that is, in particular in tissue deformation, is completely ignored,” said Ming Guo, PhD, associate professor of mechanical engineering at MIT. “Now we really show we can observe this flow. And as the tissue deforms, flow between cells dominates the behavior. So, let’s pay attention to this when we study diseases and engineer tissues.”
The team envisions the results informing the design of artificial tissues and organs. For example, in engineering artificial tissue, scientists might optimize intercellular flow within the tissue to improve its function or resilience. The researchers suspect that intercellular flow could also be a route for delivering nutrients or therapies, either to heal a tissue or eradicate a tumor.
Guo is co-senior and co-corresponding author of the team’s published paper in Nature Physics, titled “Intercellular flow dominates the poroelasticity of multicellular tissues.” The study authors include lead author Fan Liu, PhD, at MIT, along with Bo Gao, PhD, and Hui Li, PhD, of Beijing Normal University, and Liran Lei, PhD and Shuainan Liu, PhD, of Peking Union Medical College.
“Multicellular systems, including soft tissues and tumors, are composed of cells, extracellular matrix (ECM) and fluid,” the authors wrote. The tissues and organs in our body are constantly undergoing physical deformations, from the large stretch and strain of muscles during motion to the small and steady contractions of the heart. “The mechanical characteristics of cells and extracellular matrices—such as elasticity, surface tension and viscosity—can influence diseases such as fibrosis and tumor metastasis,” the team further commented.
In some cases, how easily tissues adapt to deformation can relate to how quickly a person can recover from, for example, an allergic reaction, a sports injury, or a brain stroke. “Large deformation and adaption of the tissues and organs can also happen rapidly, such as during heart beating, skeletal muscle contraction, sports injuries (for example, ligaments), allergic oedema and brain stroke,” they noted.
Exactly what sets a tissue’s response to deformation is largely unknown. And as the authors pointed out, “Multicellular tissues have traditionally been modeled as viscoelastic materials, which overlooked the abundance of intercellular space and intercellular flow within the structure.”
Guo and his group at MIT set out to look into the mechanics of tissue deformation, and the role of intercellular flow in particular, following a study they published in 2020. In that study, the researchers focused on tumors and observed the way in which fluid can flow from the center of a tumor out to its edges, through the cracks and crevices between individual tumor cells. They found that when a tumor was squeezed or pressed, the intercellular flow increased, acting as a conveyor belt to transport fluid from the center to the edges. Intercellular flow, they found, could fuel tumor invasion into surrounding regions.
For their newly reported study the team looked to see what role this intercellular flow might play in other, noncancerous tissues. “Whether you allow the fluid to flow between cells or not seems to have a major impact,” Guo commented. “So we decided to look beyond tumors to see how this flow influences how other tissues respond to deformation.”
The scientists studied intercellular flow in a variety of biological tissues, including cultured cell 3D cell spheroids and cells derived from mouse pancreatic tissue. They carried out experiments in which they first cultured small clusters of tissue, each measuring less than a quarter of a millimeter wide and numbering tens of thousands of individual cells. They placed each tissue cluster in a custom-designed testing platform that the team built specifically for the study.
“These microtissue samples are in this sweet zone where they are too large to see with atomic force microscopy techniques and too small for bulkier devices,” Guo noted. “So, we decided to build a device.”
The researchers adapted a high-precision microbalance that measures minute changes in weight. They combined this with a step motor that is designed to press down on a sample— applying parallel plate compression (PPC)—with nanometer precision. The team placed tissue clusters one at a time on the balance and recorded each cluster’s changing weight as it relaxed from a sphere into the shape of a pancake in response to the compression. The team also took videos of the clusters as they were squeezed.
For each type of tissue, the team made clusters of varying sizes. They reasoned that if the tissue’s response is ruled by the flow between cells, then the bigger a tissue, the longer it should take for water to seep through, and therefore, the longer it should take the tissue to relax. It should take the same amount of time, regardless of size, if a tissue’s response is determined by the structure of the tissue rather than fluid.
Over multiple experiments with a variety of tissue types and sizes, the team observed a similar trend: the bigger the cluster, the longer it took to relax, indicating that intercellular flow dominates a tissue’s response to deformation. “By building a tailored micro-mechanics platform to perform PPC, we find that both cultured 3D multicellular spheroids and natural mouse pancreatic islets exhibit apparent poroelasticity at a timescale of tens of seconds,” they stated. “This behavior is dominated by the interstitial fluid flow through the intercellular space between cells.… we demonstrate that the force relaxation speed depends on the interstitial fluid flow inside the multicellular tissues; a larger intercellular space or a stiffer multicellular skeleton can result in a faster fluid flow within the tissue and hence a quicker force relaxation.”
Liu added, “We show that this intercellular flow is a crucial component to be considered in the fundamental understanding of tissue mechanics and also applications in engineering living systems.”
In their paper the team also stated, “Our results highlight the critical need to consider interstitial fluid flow in understanding the structural and mechanical behaviors of multicellular systems and may lead to the discovery of potential physical regulators of development and diseases, as well as new strategies for engineering multicellular living systems.”
Going forward, the researchers plan to look into how intercellular flow influences brain function, particularly in disorders such as Alzheimer’s disease. “Intercellular or interstitial flow can help you remove waste and deliver nutrients to the brain,” Liu commented. “Enhancing this flow in some cases might be a good thing.”
Guo commented, “As this work shows, as we apply pressure to a tissue, fluid will flow. In the future, we can think of designing ways to massage a tissue to allow fluid to transport nutrients between cells.”