In the realm of neurodegenerative research, a new frontier is rapidly emerging that bridges the gap between late-life psychiatric conditions and the early biological stages of diseases such as Alzheimer’s. A groundbreaking study led by Drs. Shin Kurose and Keisuke Takahata at Japan’s National Institutes for Quantum Science and Technology (QST) reveals that mood disorders arising in later life, particularly late-onset depression and bipolar disorder, may not merely be isolated mental health issues. Instead, they could represent prodromal signs of neurodegenerative pathology, detectable well before the classical cognitive impairments of dementia manifest.
For years, clinicians and neuroscientists have recognized a perplexing association: elderly patients presenting with mood disturbances often develop neurodegenerative diseases within subsequent years. However, the causal and mechanistic links underpinning this phenomenon remained elusive, largely due to technological limitations in detecting key pathological proteins involved in brain degeneration. Prior studies predominantly explored late-life depression in relation to Alzheimer’s, leaving disorders such as late-onset bipolar disorder under-investigated.
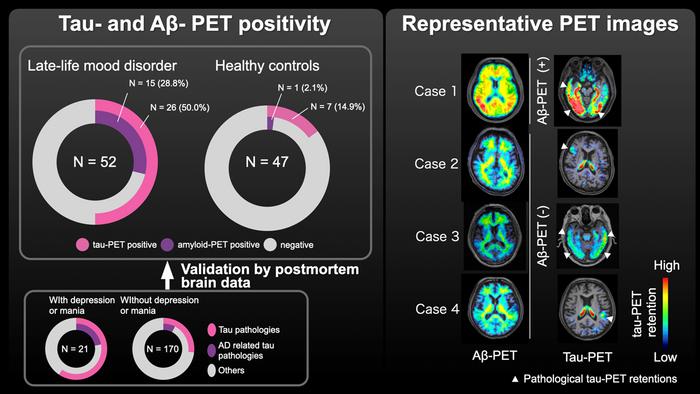
Addressing these gaps, Kurose, Takahata, and their multidisciplinary team embarked on an ambitious investigation employing sophisticated positron emission tomography (PET) imaging coupled with postmortem brain analyses. Their research utilized novel radiotracers capable of binding selectively to abnormal tau aggregates and amyloid beta deposits—hallmarks of Alzheimer’s pathology and various tauopathies. These tracers enabled visualization of pathological protein accumulations in vivo, providing a comprehensive snapshot of the neurochemical landscape in individuals with late-life mood disorders (LLMDs).
.adsslot_HgcZYehpn5{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_HgcZYehpn5{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_HgcZYehpn5{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The study cohort consisted of 52 individuals diagnosed with late-life depression or bipolar disorder and a control group of 47 cognitively healthy older adults. PET scans revealed that nearly half of the LLMD participants harbored elevated tau pathology, predominantly localized in the frontal cortex—an area instrumental for emotional regulation and executive function. By contrast, only approximately 15% of healthy controls exhibited comparable tau deposition. Amyloid beta accumulation demonstrated a similar trend: 29% in LLMD individuals versus a mere 2% among controls.
Intriguingly, these pathological changes emerged despite the absence of significant cognitive decline in most subjects, suggesting that tau and amyloid accumulation linked to mood disorders precede overt dementia symptoms by several years. This crucial insight was corroborated through analysis of 208 autopsy cases, which illuminated a consistent temporal pattern whereby mood disturbances anticipated cognitive and motor decline by an average of 7.3 years.
This research not only redefines the clinical interpretation of late-life mood disorders but also underscores the potential of tau-PET imaging as a biomarker for early neurodegenerative processes. Unlike conventional imaging modalities, which struggle to differentiate between diverse tau species, the advanced tracers used in this study demonstrated unprecedented sensitivity for detecting the heterogeneous tau pathologies implicated in dementia and related disorders.
The implications extend far beyond diagnosis. Identifying tau and amyloid pathology in patients presenting with LLMDs could pave the way for early therapeutic intervention with disease-modifying agents, potentially altering the trajectory of neurodegeneration before irreversible damage occurs. The findings advocate for integrative clinical assessments where neuropsychiatric symptoms in the elderly trigger more nuanced evaluations, including molecular imaging and possibly cerebrospinal fluid analysis.
Complementing the imaging data, the histopathological examination of autopsy specimens uncovered a spectrum of tauopathies associated with mood disorder history, ranging from Alzheimer’s-type neurofibrillary tangles to non-Alzheimer’s tau inclusions. This diversity of tau pathology challenges the traditional amyloid-centric view of dementia and invites further exploration into tau’s multifaceted role in psychiatric and neurodegenerative diseases.
The frontal lobe predilection of tau accumulation observed in mood disorder patients aligns with the neuroanatomical substrates governing cognition and affective control. Disruptions within these circuits may therefore underlie the early psychiatric manifestations, providing a neuropathological explanation for the clinical presentation of LLMDs as sentinel events heralding more profound neurodegeneration.
Dr. Kurose emphasized that the study’s robust methodology and multimodal approach—combining state-of-the-art PET imaging with extensive postmortem validation—represent a significant leap in understanding the continuum from mood disorders to dementia. The ability to detect pathological hallmarks in living patients holds promise for refining diagnostic criteria and personalizing treatment strategies.
Moreover, Dr. Takahata highlighted the prospective value of the novel PET tracers utilized, underscoring their capacity to discern multiple tau variants. This capability not only enhances diagnostic precision but also enriches our comprehension of disease heterogeneity, which is vital for the development of targeted therapies.
The social and clinical burden posed by neurodegenerative diseases continues to escalate globally, intensifying the urgency for novel diagnostic tools and early intervention frameworks. By illuminating a previously underappreciated prodromal window manifesting as mood disorders, this study reframes late-life psychiatric symptoms within the broader context of neurobiological decline.
Looking forward, the integration of molecular imaging into routine psychiatric evaluations could transform clinical paradigms, enabling stratification of patients based on underlying neuropathology rather than solely symptomatology. Such an approach promises to enhance prognostic accuracy and facilitate timely deployment of emerging disease-slowing treatments.
This pioneering work thus stands at the intersection of psychiatry, neurology, and molecular imaging, advocating for a paradigm shift in how late-life mood disorders are perceived and managed. It opens fertile ground for future research into the molecular triggers of neurodegeneration and the development of sensitive diagnostic platforms capable of capturing the earliest pathological changes in the human brain.
In summary, the study by Kurose, Takahata, and colleagues offers compelling evidence that late-life depression and bipolar disorder are not merely psychiatric diagnoses but may be early indicators of diverse tauopathies and Alzheimer’s-related pathology. This paradigm-challenging revelation invites the scientific community and clinicians alike to reconsider diagnostic and therapeutic strategies, with the hopeful prospect of intercepting neurodegenerative diseases at their nascent stages.
Subject of Research: People
Article Title: Diverse tau pathologies in late-life mood disorders revealed by PET and autopsy assays
News Publication Date: 9-Jun-2025
References: DOI: 10.1002/alz.70195
Image Credits: Dr. Keisuke Takahata from the National Institutes for Quantum Science and Technology, Japan
Keywords: Dementia, Cognitive disorders, Affective disorders, Neurodegenerative diseases, Medical diagnosis, Positron emission tomography, Alzheimer disease, Tau proteins, Mental health, Amyloids
Tags: Alzheimer’s disease risk factorsamyloid beta depositsbipolar disorder and dementiaearly onset dementialate-life mood disorderslate-onset depressionmental health and agingmultidisciplinary research in psychiatryNeurodegenerative disease researchPET imaging in neuroscienceprodromal signs of dementiatau protein aggregates