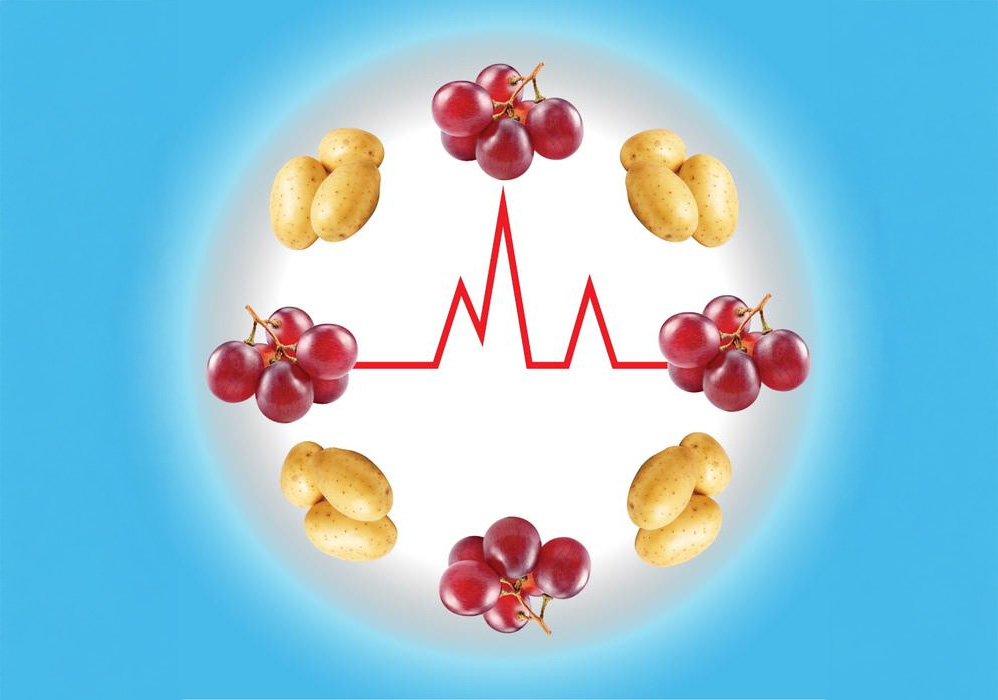
![Stanford_Food_MetabolicHealth Study participants' blood glucose responses to foods such as grapes and potatoes varied depending on their metabolic dysfunction. [Emily Moskal/Stanford Medicine]](https://www.genengnews.com/wp-content/uploads/2025/06/Stanford_Food_MetabolicHealth-696x488.jpg)
A study led by researchers at Stanford Medicine has shown that differences in blood sugar responses to certain carbohydrates depend on details of an individual’s metabolic health status, including specific metabolic conditions such as insulin resistance or beta cell dysfunction, both of which can lead to diabetes. The findings indicate that this variability in blood sugar response could lead to personalized prevention and treatment strategies for prediabetes and diabetes.
“Right now, the American Diabetes Association dietary guidelines do not work that well because they lump everyone together,” said Michael Snyder, PhD, the Stanford W. Ascherman, MD, FACS Professor in Genetics. “This study suggests that not only are there subtypes within prediabetes, but also that your subtype could determine the foods you should and should not eat.”
Snyder is senior author of the researchers’ published paper in Nature Medicine, titled “Individual variations in glycemic responses to carbohydrates and underlying metabolic physiology.” Tracey McLaughlin, MD, a professor of endocrinology, co-led the study with Snyder. Joint first authors are Yue Wu, PhD, a postdoctoral scholar in genetics, Ben Ehlert, a graduate student in biomedical data science, and Ahmed Metwally, PhD, a former postdoctoral scholar at Stanford Medicine who is now a research scientist at Google, are joint first authors.
The authors cited figures indicating that one in three adults in the United States has prediabetes, and 70% of these individuals will develop type 2 diabetes (T2D), which poses a substantial public health burden via complications such as kidney disease, vision loss, neuropathy, cardiovascular disease (CVD), and cancer. “High postprandial glycemic responses (PPGRs) are a hallmark of prediabetes and T2D and are risk factors for T2D, CVD, and all-cause mortality independent of fasting blood glucose (FBG) and HbA1c,” they explained. “However, our understanding of glucose dysregulation, especially regarding PPGRs, remains incomplete.”
There is more than one pathway to diabetes, which is currently diagnosed based on elevated blood sugar levels, known as hyperglycemia. Beta cells in the pancreas make the hormone insulin, which is then distributed to cells throughout the body to help convert glucose in the blood into energy. Beta cell dysfunction occurs when the pancreas fails to make or to release enough insulin, and insulin resistance occurs when cells in the body do not respond fully to insulin. Both beta cell dysfunction and insulin resistance can contribute to the high blood sugar levels that define prediabetes and type 2 diabetes. The authors further noted, “A gap in understanding how underlying metabolism and physiology affect PPGRs exists largely because quantifying metabolic functions, such as insulin resistance, insulin secretion, and the incretin effect, is laborious and costly and has not been extensively employed.”
For their reported study, the team measured PPGRs using continuous glucose monitoring in 55 participants without a history of type 2 diabetes, in response to seven different standard carbohydrate meals. The participants underwent metabolic testing for insulin resistance and beta cell dysfunction in addition to multi-omics profiling, which included tests for triglyceride levels, metabolites in plasma of the blood, measures of liver function, and gut microbiome data. “We performed gold-standard metabolic tests and multi-omics profiling to examine the physiologic and molecular basis for interindividual PPGR differences,” the team explained. Twenty-six of the 55 participants had prediabetes.
For the study, the participants wore continuous glucose monitors (CGMs) and ate the same-sized portions of different carbohydrates that were delivered to their homes. The seven foods tested were jasmine rice, buttermilk bread, shredded potato, pasta, canned black beans, grapes, and a berry mix containing blackberries, strawberries, and blueberries. The participants consumed the food first thing in the morning, after fasting for 10 to 12 hours. Each participant ate each food type twice, and the research team tracked the individual’s blood sugar response during the three hours after their meal. “We hypothesized that individual PPGRs are associated with the underlying metabolic physiology (for example, insulin resistance and beta cell dysfunction) as well as molecular markers,” the team stated.
The results showed that many participants had a blood glucose spike after eating rice or grapes, regardless of their metabolic health status. The blood glucose responses to foods containing the highest amounts of resistant starch—potatoes and pasta—varied depending on the participants’ metabolic dysfunction. “Starchy foods were not equal; there was a lot of individual variability in which foods produced the highest glucose spike,” Wu said.
The highest blood sugar spikes after eating pasta occurred in participants who had insulin resistance, and the highest spikes after eating potatoes occurred in participants who were either insulin resistant or had beta cell dysfunction. Multi-omics profiling showed that the potato-spiking participants also had high levels of triglycerides, fatty acids, and other metabolites commonly seen in people with insulin resistance.
Glucose spike responses to beans were associated with histidine and keto metabolism, a state in which the body primarily uses fat for energy. Participants whose blood sugar spiked after eating bread were more likely to have hypertension, or high blood pressure. “Overall, rice was the most glucose-elevating carbohydrate meal, but there was considerable interindividual variability,” the authors further reported. “Rice-spikers were more likely to be Asian individuals, and bread-spikers had higher blood pressure.”
The highest blood glucose spikes after eating potatoes occurred in the participants who were the most insulin resistant and had the lowest beta cell function. Everyone spiked to some extent after eating grapes. “Individuals with the highest PPGR to potatoes (potato-spikers) were more insulin resistant and had lower beta cell function, whereas grape-spikers were more insulin sensitive,” the team further noted. The comparison of the blood glucose responses to potatoes versus grapes was associated with having insulin resistance, suggesting that this ratio could serve as a real-world biomarker for insulin resistance in the future. “Such a biomarker would be useful because insulin resistance is amenable to lifestyle and medication interventions that can reduce risk for diabetes in high-risk individuals,” McLaughlin said. “At present, there is no easy way to diagnose it in the clinic.”
The researchers also examined whether eating a portion of fiber, protein, or fat before carbohydrates reduced blood sugar spikes. “We also examined whether preloading a rice meal with fiber, protein, or fat (‘mitigators’) altered PPGRs,” they explained. For this part of the study, participants ate pea fiber powder, protein from boiled egg whites, or fat in the form of crème fraîche 10 minutes before eating rice.
The results showed that eating fiber or protein before the rice lowered the glucose spike, and eating fat before the rice delayed the peak of the spike. But these changes in blood glucose response occurred only in the metabolically healthy participants who were insulin sensitive or had normal beta cell function. “Mitigators were less effective in reducing PPGRs in insulin-resistant as compared to insulin-sensitive participants,” the investigators stated.
And while it was found that eating fat, protein, or fiber before carbohydrates had minimal impact on the blood glucose response patterns in participants with insulin resistance or beta cell dysfunction, McLaughlin and Snyder both think this type of mitigation is worth further research. “Eating carbohydrates later in a meal is still a good idea, even though it has not yet been sorted out whether it is best to eat protein, fat, or fiber before carbohydrates. Eat your salad or hamburger before your French fries,” Snyder recommended.
“We found associations between PPGRs and blood glucose and omics features, and their relationship to the specific metabolic dysfunctional phenotype, including insulin resistance and beta cell function, enabling a deeper understanding of interindividual variation in PPGRs,” the authors wrote in summary. Noting limitations of their study, they concluded, “Overall, our results represent an initial step in evaluating the effect of different meals and mitigators on PPGRs and their associations with metabolic physiology, especially because individuals, both healthy and at risk, have increased access to CGMs to optimize health by modifying their diet.”