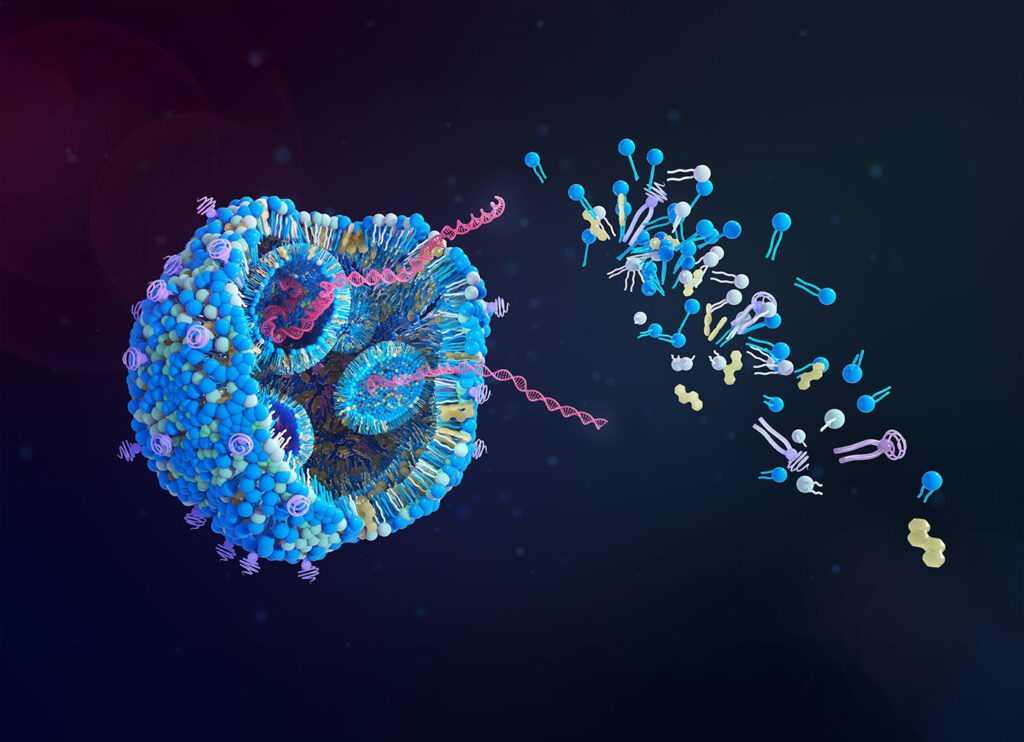
In recent years, mRNA technology has come into its own, most prominently in the field of vaccine development. After mRNA’s success in the COVID-19 vaccine, companies are now harnessing the molecule to tackle some of the world’s most challenging pathogens, including not only viruses but bacteria as well, such as antibiotic-resistant gonorrhea and the variety of E. coli that causes recurrent urinary tract infection. Companies including CureVac, Afrigen Biologics, Moderna, and Pfizer/BioNTech are leveraging cutting-edge mRNA platforms to focus on prevention of these often-intractable diseases, and they have promising preclinical and clinical results.
A better way to fight recurrent UTIs
CureVac, based in Tubingen, Germany, and a pioneer in mRNA technology since 2000, has multiple mRNA vaccines in their pipeline targeting respiratory and non-respiratory infectious diseases. They have collaborated with GSK on respiratory viruses since 2020, and in July 2024 announced a licensing agreement under which GSK takes over development and manufacturing of vaccines for seasonal influenza, avian influenza, and COVID-19, as well as a combination flu/COVID-19 vaccine.

CureVac’s non-respiratory infectious disease program targets recurrent urinary tract infections (UTIs) caused by uropathogenic E. coli (UPEC). The mRNA vaccine targets the fimH antigen, an adhesive surface protein that helps the bacteria colonize the bladder and kidney. UPEC is responsible for 70-90% of recurrent UTIs, and the fimH antigen is highly conserved and stable, explained Alexander Zehnder, MD, CEO of CureVac. “It’s an ideal setup for a vaccine,” he said. “You have a bacteria that’s the main culprit, and you have a target which is extremely stable.”
In preclinical studies, CureVac’s mRNA vaccine performed better than a protein-based comparator both in terms of generating antibodies against fimH and in stimulating a T-cell response, a key step in preventing recurrent infections. Anti-fimH antibodies will prevent UPEC bacteria from infecting the enteric wall, Zehnder noted, but they can’t help cells that have already been infected. “The beauty of mRNA is that it does more than that,” he said. “Not only can you provide antibodies to prevent the infection, but you can stimulate the immune system with the T cells, and the T cells attack already infected cells.”
Zehnder said that CureVac plans to file an Investigational New Drug (IND) application in the second half of 2025 and start phase I clinical trials in early 2026. If successful, the vaccine could help cut down on the use of antibiotics for these persistent infections. “People with recurrent infections could have antibiotic treatments multiple times a year,” Zehnder said. Avoiding repeated antibiotic use not only reduces the risk of creating antibiotic-resistant bacteria but also frees patients from the unpleasant side effects that often accompany antibiotic use.
Taking on antibiotic-resistant gonorrhea
South Africa–based Afrigen Biologics, in collaboration with Danish biotech firm Evaxion, has demonstrated preclinical proof-of-concept for an mRNA vaccine for gonorrhea, a sexually transmitted bacterial infection caused by the bacteria Neisseria gonorrhoeae. Although there are effective antibiotics to treat gonorrhea, antimicrobial resistant strains are on the rise, making the disease increasingly dangerous globally. The vaccine, EVX-B2, is based on antigens identified by Evaxion’s artificial intelligence platform, AI-Immunology™. A protein-based version of EVX-B2, developed at the University of Massachusetts, showed significant immunogenicity and bactericidal activity in preclinical testing. Afrigen collaborated on the adjuvant for that protein-based vaccine.

During the COVID-19 pandemic, Afrigen began manufacturing mRNA vaccines, and in 2021 the company became the hub of the mRNA Vaccine Technology Transfer Programme, a global initiative by the World Health Organization and the Medicines Patent Pool to build vaccine manufacturing capacity in low- and middle-income countries. After the pandemic, Afrigen turned their attention back to gonorrhea, this time with mRNA in mind. “COVID has proven mRNA is a suitable platform for safe and effective vaccine development,” said Petro Terblanche, PhD, CEO of Afrigen. So far, however, no mRNA vaccine has been approved for clinical use against a bacterial target. Afrigen and Evaxion began working on an mRNA version of the EVX-B2 gonorrhea vaccine.
Preclinical data have shown that the mRNA version of the antigen performs comparably to the protein antigen, demonstrating potential for robust immune response. “This is early stage, but incredibly promising,” said Terblanche. “This is a vaccine that has global relevance.” The next steps will be to continue the pre-clinical development, file an IND application and scale up production for clinical trials. If the mRNA vaccine is effective, it would represent a major public health advance against gonorrhea, a disease the CDC has named as one of the most urgent antibiotic-resistant disease threats in the United States.
Going with the gut
Moderna began a phase III clinical trial in September 2024 to evaluate the safety and efficacy of their mRNA vaccine for norovirus, a highly contagious virus that causes gastroenteritis. The trial will enroll around 25,000 participants, 80% of whom will be over 60 years old. “We’re really focusing on this older age group because, at least in adults, the greatest burden of norovirus disease is in elderly individuals,” said Doran Fink, MD, PhD, clinical therapeutic area head for gastrointestinal and bacterial pathogens at Moderna. “They account for most of the hospitalizations and deaths caused by norovirus acute gastroenteritis in adults in the U.S.” The trial is currently enrolling participants in the United States, the United Kingdom, Canada, and Japan, and will soon expand to Australia and Latin America.
The benefit of using the mRNA platform for norovirus is its capacity for speedy updates as new variants arise. Studies of natural infection show that people can get infected with norovirus multiple times, both because immune protection wanes over time and because there are so many different genotypes of norovirus. “If a new genotype becomes predominant, it’s possible that the vaccine might need to be updated,” said Fink. “That’s one of the advantages of our mRNA platform that we’re hoping to harness, the ability to rapidly update the vaccine composition over time as needed.” If the vaccine program is successful, Fink said, Moderna would work with public health authorities to coordinate vaccine production with surveillance data regarding which variants are circulating. The vaccine being tested is a trivalent formulation against three common norovirus genotypes. “Our vaccine includes the GII.4 genotype and two other additional genotypes that have, on average, been most predominant over the last decade or so,” Fink says.
The norovirus vaccine is based on Moderna’s Spikevax COVID-19 vaccine and uses the same mRNA and lipid nanoparticle technology. Another norovirus vaccine currently in development by Takeda Pharmaceuticals spinout HilleVax, using virus-like particles rather than mRNA, failed a phase II trial in infants in July after showing promising results in adults. Moderna’s vaccine would similarly need to show efficacy in children and infants to satisfy vaccine licensure requirements. “There’s a big public health need for vaccines to prevent norovirus in infants and children, especially in lower- and middle-income countries, so the regulatory obligation wouldn’t be the only reason for developing this,” Fink said.
Combination virus for respiratory disease
Pfizer and BioNTech have released results from a phase III clinical trial of their combination COVID-19 and influenza vaccine. The vaccine includes the company’s licensed COVID-19 vaccine combined with an mRNA-based influenza vaccine candidate. The goal is to simplify immunization processes by reducing the number of injections required for respiratory virus protection, potentially increasing vaccination rates and public health outcomes.
The trial compared the immunogenicity of the combination vaccine with the current standard of care, licensed COVID-19 and influenza vaccines given in opposite arms at the same visit. Results showed that the combination vaccine elicited antibody responses comparable to the licensed COVID-19 vaccine, and higher responses against influenza A. However, the combination vaccine candidate did not outperform the licensed vaccine against influenza B, and the companies are evaluating adjustments aimed at improving that immune response. “The apparent attenuation of immunogenicity responses to influenza B immunogenicity has been observed in other influenza vaccines besides mRNA,” said Kit Longley, a spokesperson for Pfizer, by email. “The clinical significance of these immunogenicity results is less clear.”
Pfizer also announced data from a phase II trial of its second-generation trivalent influenza vaccine, which showed robust immunogenicity against all strains compared with standard of care. “The immunogenicity data from our phase II study of the trivalent mRNA standalone influenza vaccine gives us confidence that we are on the right track to usher in the next generation of investigational mRNA influenza and COVID-19 combination vaccine candidates,” Longley said.