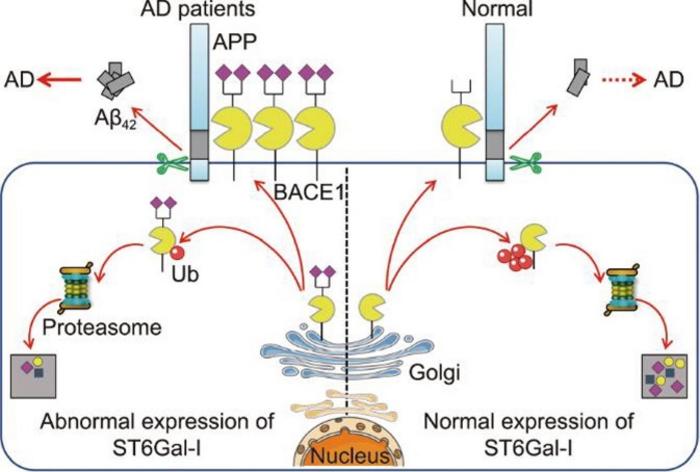
A recent groundbreaking study published in the esteemed journal Engineering sheds new light on the intricate biochemical mechanisms underlying Alzheimer’s disease (AD). Researchers have made significant strides in unraveling the complex role of α2,6-sialylation, a critical post-translational modification mediated by the enzyme sialyltransferase-I (ST6Gal-I). This work investigates how alterations in glycosylation pathways influence the expression of β-site amyloid precursor protein cleaving enzyme 1 (BACE1), a key player in the generation of amyloid-β (Aβ) plaques. These plaques are hallmarks of AD, marking the degenerative changes that characterize this devastating condition.
Understanding the biochemical landscape of Alzheimer’s Disease is vital, given the escalating global incidence of this neurodegenerative disorder. The amyloidogenic hypothesis has long posited that Aβ plaque deposition is a primary driver of pathogenesis in AD, emphasizing the need for effective therapeutic interventions. As BACE1 catalyzes the production of Aβ peptides through its cleavage of the amyloid precursor protein (APP), its regulation is paramount. The newly published study highlights the pivotal role of ST6Gal-I in modulating BACE1 expression and, consequently, the progression of amyloid pathology.
The researchers embarked on a comprehensive investigation across various experimental models, including human cerebrospinal fluid, serum samples from AD patients, and genetically engineered AD model mice. They discovered stark elevations in both ST6Gal-I expression and α2,6-sialylation levels in all affected samples, suggesting a significant correlation between increased sialylation and AD pathology. This finding draws attention to the often-overlooked aspect of glycosylation and its implications for neurodegenerative diseases.
.adsslot_0s2MHeKh5v{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_0s2MHeKh5v{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_0s2MHeKh5v{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Further elucidating this relationship, the research team employed CRISPR/Cas9 gene-editing technology to create ST6Gal-I knockout rats. These genetically modified rats exhibited a notable decrease in α2,6-sialylation levels and lower BACE1 expression in brain tissues. Notably, behavioral assessments conducted through various cognitive tests indicated that ST6Gal-I knockout rats experienced significantly less cognitive impairment when subjected to scopolamine, a pharmacological agent utilized to model memory deficits akin to those seen in AD. These outcomes suggest that the absence of ST6Gal-I can provide a protective effect against cognitive decline.
Investigating the mechanistic basis of these findings further, the researchers demonstrated that knockdown of ST6Gal-I in Neuro-2a neuroblastoma cells led to enhanced ubiquitination of BACE1. This increased ubiquitination marked BACE1 for degradation, resulting in reduced levels of amyloid-beta production and lowered cell apoptosis. The interplay of glycosylation and protein turnover in the context of AD raises compelling questions regarding potential therapeutic avenues targeting ST6Gal-I to modulate cognitive decline.
This line of inquiry extends the frontier of glycomedicine, revealing the biochemical richness and compositional heterogeneity of glycosylation in health and disease. As the body of evidence supporting the connection between glycosylation patterns and the pathological hallmarks of Alzheimer’s disease continues to expand, researchers are urged to consider the implications of targeting glycosylation pathways in developing effective treatments.
Moreover, engaging with the complex signaling pathways affected by glycosylation brings forth exciting opportunities for intervention. Identifying key molecular targets along these pathways may unveil novel strategies to halt or even reverse cognitive decline in Alzheimer’s patients. The comprehensive understanding afforded by this research brings us closer to realizing a multifaceted approach to AD treatment that takes into account the critical role of glycosylation.
Going forward, the implications of these findings warrant further exploration. Future studies could delve into the molecular mechanisms of α2,6-sialylation regulation and its broader effects on neuronal health or explore the potential for pharmacologic or genetic modulation of these pathways. By pinpointing the biochemical pathways that govern ST6Gal-I activity, researchers can set the stage for innovative therapeutic approaches designed to combat Alzheimer’s disease effectively.
In conclusion, the insights gained from this multifaceted study provide new hope in the relentless pursuit of effective Alzheimer’s therapies. By illuminating the previously obscure role of glycosylation in the pathophysiology of AD, this research represents a significant leap forward in our understanding of this complex disease. The future may hold promising advancements as the scientific community continues to unravel the complexities of glycosylation and its role in neurodegeneration.
The collaborative efforts of the research team, which includes esteemed institutions such as the Cancer Hospital of Shantou University Medical College and the Institute for Genome Engineered Animal Models of Human Diseases, emphasize the multidisciplinary approach needed to tackle such intricate biological questions. As we continue to expand our understanding, it becomes essential to share these findings widely, fostering collaboration and discussion within the scientific community. The quest for effective treatment options for Alzheimer’s disease is more critical than ever, and this research marks an important step towards that goal.
Through advancements in genetic editing technology and a deeper understanding of glycosylation’s role, we find ourselves at the forefront of potentially transformative strategies in Alzheimer’s research. As this field evolves, maintaining focus on the intricate biological networks at play will be vital to developing targeted interventions that can mitigate the impact of one of the world’s most pressing health challenges.
Subject of Research: The Role of α2,6-Sialylation in Alzheimer’s Disease
Article Title: Ablation of ST6Gal-I Downregulates BACE1 Expression and Suppresses Production of Aβ42 Plaques in Alzheimer’s Disease
News Publication Date: [Insert Date]
Web References: [Insert URL]
References: [Insert relevant references]
Image Credits: Kangkang Yang, Xueying Li, Minchao Lai, Weiwei Zhao, Wanli Song, Shaobin Chen, Wenzhe Li
Keywords
Alzheimer’s Disease, α2,6-Sialylation, ST6Gal-I, BACE1, Amyloid Plaques, Cognitive Impairment, Glycosylation, Neurodegeneration, CRISPR/Cas9, Sialyltransferase, Biomarkers, Therapeutics.
Tags: 6-sialylation in Alzheimer’s diseaseadvancements in neurodegAlzheimer’s research and experimental modelsamyloidogenic hypothesis and AD pathogenesisBACE1 expression and amyloid plaquesbiochemical mechanisms of Alzheimer’s diseasecerebrospinal fluid analysis in Alzheimer’s patientsglycosylation pathways in Alzheimer’simpact of post-translational modifications in ADregulation of amyloid precursor protein cleavagerole of sialyltransferase-I in neurodegenerationtherapeutic interventions for Alzheimer’s diseaseα2