A groundbreaking study published in Genes & Diseases has unveiled critical insights into how the aging process and the molecule EMP1 collaboratively drive the progression of resectable pancreatic cancer (PC). Conducted by a team of researchers from the University of Chinese Academy of Sciences and Chongqing Medical University, this work establishes a novel prognostic framework that connects EMP1 expression levels with worsened clinical outcomes, especially in elderly patients. This research not only broadens our understanding of tumor biology in the context of aging but also opens new avenues for targeted therapeutic intervention.
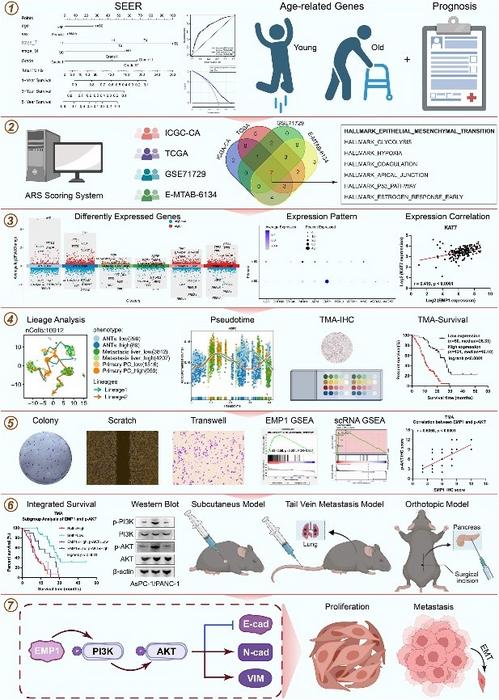
Pancreatic cancer, notorious for its aggressive nature and poor prognosis, presents a significant challenge in oncology, particularly when tumors are amenable to surgical resection. The new study places emphasis on the biological interplay between systemic aging and the tumor microenvironment, highlighting how age-related physiological changes potentiate tumor proliferation and metastatic spread. Central to their approach is the development of an Age-Related Score (ARS) system, which integrates large-scale bulk RNA sequencing and single-cell RNA sequencing data to stratify postoperative pancreatic cancer patients according to their prognostic risk.
One of the pivotal discoveries of the study is the identification of Epithelial Membrane Protein 1 (EMP1) as a key molecular player influencing pancreatic cancer progression. Empirical data revealed a robust correlation between high EMP1 levels and decreased survival rates, suggesting that EMP1 acts as an oncogenic driver within the aged tumor milieu. This aligns with emerging paradigms positing that aging-associated molecular pathways deepen tumor aggressiveness and resistance to therapy.
.adsslot_83FSU0KOm9{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_83FSU0KOm9{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_83FSU0KOm9{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Delving deeper into mechanistic underpinnings, the research team demonstrated how EMP1 modulates cellular behavior through the activation of the PI3K/AKT signaling cascade, a well-established pathway implicated in cell growth, survival, and motility. Both in vitro cellular models and in vivo mouse models showed that elevated EMP1 expression fosters increased cellular proliferation, migration, and invasion — hallmarks of malignant progression. The use of cellular trajectory analyses further illuminated how elevated ARS scores and EMP1 overexpression coincide with heightened epithelial-mesenchymal transition (EMT) processes, advancing tumor invasiveness.
The translational relevance of these findings was strengthened via mouse models simulating varying degrees of pancreatic cancer progression. Utilizing subcutaneous models, pulmonary metastasis assays, and orthotopic pancreatic liver metastasis systems, the study revealed that suppression of EMP1 expression markedly reduced tumor burden and metastatic dissemination. Conversely, EMP1 overexpression accelerated tumor growth and spread, underscoring the molecule’s potential as a therapeutic target.
Importantly, the study demonstrated that pharmacological inhibition of the PI3K/AKT pathway through LY294002 effectively reversed EMP1-induced oncogenic effects. This pharmacologic intervention not only curtailed tumor proliferative capacity but also mitigated metastatic tendencies, highlighting a viable strategy to disrupt EMP1-mediated signaling in clinical scenarios. The implications of this therapeutic avenue could be transformative for pancreatic cancer patients, particularly for those considered high risk based on ARS assessments.
Furthermore, by integrating bulk and single-cell RNA sequencing data, the authors have provided a high-resolution map of cellular heterogeneity within the aged pancreatic tumor microenvironment. This approach revealed that aging amplifies tumor plasticity and fosters a microenvironment conducive to tumor progression via changes in immune modulation and stromal interactions. While the study primarily focused on EMP1 and PI3K/AKT signaling, the authors suggest that further exploration of the immune landscape will be crucial for full mechanistic elucidation.
The research also sheds light on the complex nature of aging as a biological process that transcends mere chronological advancement. Aging influences cellular senescence, epigenetic alterations, and metabolic reprogramming—each of which can potentiate oncogenic pathways like the one mediated by EMP1. Understanding these multifaceted connections offers promise for developing age-specific, precision medicine approaches that can more effectively target pancreatic cancer in older patient populations.
In addition to experimental data, the research team engaged computational modeling to validate their prognostic framework, demonstrating that ARS scores are highly predictive of patient outcomes. This model, which combines molecular markers with clinical data, represents a significant step toward personalized medicine in pancreatic oncology, where stratification based on molecular aging signatures can inform treatment decisions and optimize therapeutic efficacy.
From a therapeutic innovation standpoint, targeting EMP1 and its downstream PI3K/AKT signaling represents a potential paradigm shift. Current pancreatic cancer treatments suffer from limited efficacy due to early metastasis and resistance mechanisms; thus, novel targets such as EMP1 provide a much-needed focus for drug development. The reversibility of EMP1-driven oncogenic signaling by LY294002 offers a proof-of-concept that molecularly targeted inhibitors can counteract tumor aggressiveness linked to aging biology.
The importance of this study extends beyond pancreatic cancer, as it highlights the broader theme of how aging-related molecular pathways intersect with cancer biology. Given the increasing demographic shift toward aging populations worldwide, these insights are timely and have implications for other malignancies where age is a predominant risk factor. By combining high-throughput molecular analyses with rigorous in vivo validation, this research exemplifies the future roadmap for integrating aging biology into cancer prognostication and therapy.
In conclusion, this comprehensive investigation delineates the intertwined roles of aging and EMP1 in driving pancreatic cancer progression. The study’s development of the ARS prognostic model, mechanistic elucidation of EMP1-mediated PI3K/AKT activation, and the demonstration of therapeutic reversibility with LY294002 pave the way for novel, age-informed interventions. Future research focused on the immune microenvironment and detailed mechanistic pathways will be essential to fully harness the therapeutic potential uncovered herein, ultimately aiming to improve outcomes for pancreatic cancer patients worldwide.
Subject of Research: The role of aging and EMP1 in the progression of resectable pancreatic cancer and the development of a prognostic model integrating molecular aging biomarkers.
Article Title: The role of the aging process and related factor EMP1 in promoting progression of resectable pancreatic cancer
Web References: http://dx.doi.org/10.1016/j.gendis.2024.101490
References: Junfeng Zhang, Jianyou Gu, Tao Zhang, Renpei Xia, Jianbo Li, Mingda Tan, Yongjun Yang, Jifeng Xiang, Bin Xie, Rong Tang, Wangge Li, Xianxing Wang, Shixiang Guo, Huaizhi Wang. Genes & Diseases, Volume 12, Issue 5, 2025, 101490.
Image Credits: Genes & Diseases
Keywords: Pancreatic cancer, EMP1, Aging, Prognostic model, PI3K/AKT signaling, Epithelial-mesenchymal transition, RNA sequencing, Therapeutic target
Tags: Age-Related Score system in oncologyaggressive nature of pancreatic canceraging and cancer prognosisclinical outcomes in elderly cancer patientsEMP1 and pancreatic cancer progressionmolecular mechanisms in pancreatic cancer treatmentprognostic framework for pancreatic cancersingle-cell RNA sequencing in cancer researchsystemic aging and tumor microenvironmenttargeted therapies for pancreatic cancertumor biology in aging patientsunderstanding metastatic spread in tumors