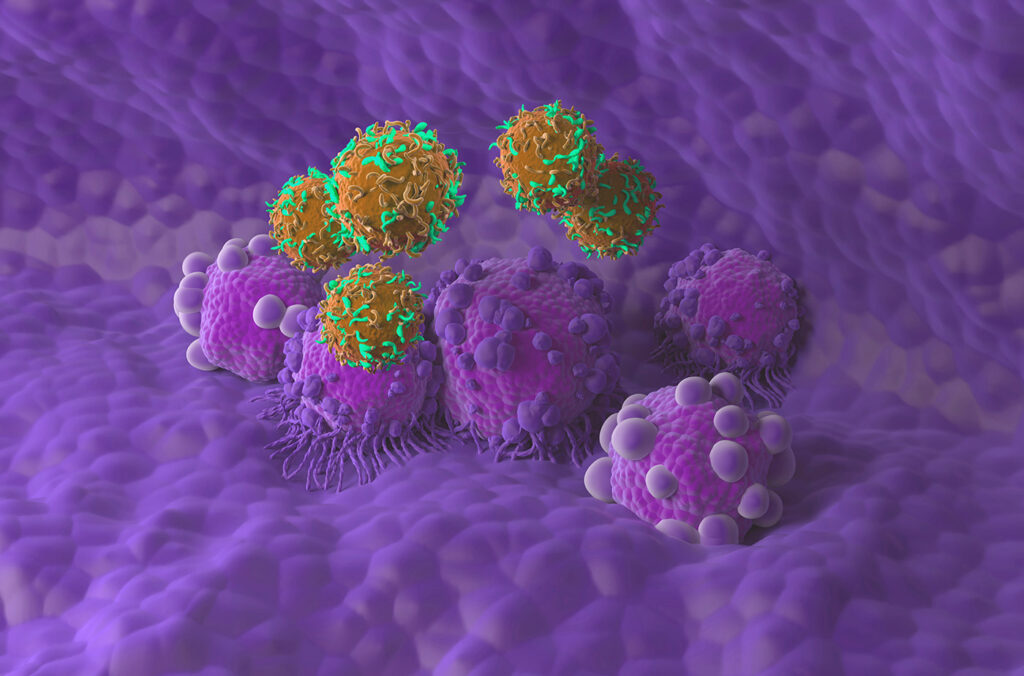
Researchers at the Keck School of Medicine of University of Southern California (USC) have developed a new type of chimeric antigen receptor (CAR) T cell that elicits a more controlled immune response to cancer in mice. Using a technology called Synthetic TCR signaling for Enhancing Memory T cells (STEM), the team created CARs with a ZAP70-derived signaling domain (ZAP327) that showed enhanced therapeutic antitumor activity and increased in vivo T cell persistence.
The researchers’ preclinical study, including tests in mouse models, demonstrated that the new CAR constructs effectively kill cancer cells, including those that typically escape detection, with fewer toxic side effects. The team says their engineered CAR T cells may in future offer a way to more safely treat blood cancers and reduce the chance of relapse. “We found that our CAR T cells can destroy cancer cells at least as well as FDA-approved CAR T therapies, but with fewer toxic side effects,” commented Xin Liu, PhD, a postdoctoral research associate in medicine at the Keck School of Medicine.
In a newly published study in Science Translational Medicine (“ZAP327 signaling domain–driven chimeric antigen receptor generates robust and long-term antitumor immunity in mouse models,”) the researchers, including first author Liu, and senior author Rong-Fu Wang, PhD, professor of medicine and pediatrics at the Keck School of Medicine, concluded, “Our ZAP327-driven CAR T cells may improve the safety and T cell persistence issues associated with current CAR T cell immunotherapy, thus developing the next generation of CAR T cell immunotherapy for both blood and solid cancers.”
CAR T cell therapy is a form of cancer treatment that modifies a patient’s own immune cells to fight cancer. It has shown great promise for treating blood cancers such as leukemia and lymphoma, but still faces some significant challenges. Approximately 30-50% of patients who receive CAR T cell therapy relapse within one year of treatment, while others have a dangerous immune reaction, known as cytokine release syndrome (CRS), or cytokine storm, which can be fatal. These problems often stem from issues such as CAR T cells not surviving long enough in the body, cancer cells becoming harder to recognize, and treatment-related toxicity.
“Chimeric antigen receptor (CAR) T cell therapy has shown impressive clinical responses in the treatment of blood cancers, but high percentages of disease relapse one year after T cell infusion and severe toxicities associated with CAR T cell therapy remain major issues,” the authors noted. “Given that T cell persistence is closely associated with therapeutic efficacy and long-term cancer regression in CAR T cell therapy, developing strategies for improving or prolonging T cell persistence is fundamentally important for T cell immunotherapy.”
CAR T cells express a receptor on the cell surface that recognizes cancer cells and signaling molecules inside the cell that activate the immune response. “Current CAR constructs typically contain four major components: an antigen-binding domain, a hinge and transmembrane domain (TM), an intracellular costimulatory domain, and a T cell signaling domain,” the team explained.
To address the safety and efficacy issues with existing CAR T therapies, the Keck School of Medicine team focused on redesigning the cell’s internal signaling machinery, using their new technology, Synthetic TCR signaling for Enhancing Memory T cells (STEM).
All CAR T cell products currently approved by FDA use the same signaling protein—CD3 zeta chain, or CD3ζ—to activate T cells for cancer destruction. While they often work well, these cells can lose strength too quickly and may not survive long in the body, which means for some patients the cancer will return. “Because of the importance of T cell signaling, we asked whether the CD3ζ chain in the CAR construct could be replaced with signaling domains derived from other signaling molecules,” the investigators noted.
To look for a safer and more effective alternative, they screened molecules involved early in the T-cell signaling process—proteins that help guide how strongly and how long T cells stay activated. One molecule, ZAP70, stood out for its ability to strongly activate CAR T cells without overstimulating them. The researchers tested several forms of the molecule and found that one piece, known as ZAP327, provided the best balance of safety and potency. The team then replaced CD3ζ with ZAP327 to create the next-generation CAR T cells.
Using mouse models the team tested the new CAR T cells, comparing them with conventional, FDA-approved CAR T cells and other recently engineered CAR T cells. The investigators found that, compared with FDA-approved CAR T cells and other new varieties, the STEM-engineered CAR T cells performed as well as or better against cancer cells and kept their cancer-fighting abilities for longer. “… ZAP327 CAR T cells showed enhanced antitumor response and prolonged mouse survival in mouse tumor models,” they stated.
Studies showed that the STEM-engineered CAR T cells outperformed conventional CAR T cells in several ways. They survived longer, remained in a healthier memory-like state that helps them survive and respond to returning cancer, and even eliminated cancer cells that typically evade detection by existing CAR T therapies. The results suggest that the STEM-engineered cells may be more effective at recognizing and preventing disease relapse after remission. “Mechanistically, we found that the ZAP327 signaling domain promoted stem-like memory T (TSCM) cells and expression of stem cell markers in ZAP327 CAR T cells, thus prolonging T cell persistence,” the investigators noted.
Importantly, STEM-engineered CAR T cells performed better against “low-antigen” cancer cells. These cancer cells escape the body’s immune response, learning to display fewer signs that they are unwelcome invaders, which makes them harder for T cells to detect and kill. “… ZAP327-driven CAR T cells outperform conventional and several recently improved CAR T cells in therapeutic antitumor immunity, particularly in an antigen-low expression tumor model, which is clinically relevant and important for immune escape and disease relapse,” the investigators pointed out.
The new CAR T cells produced fewer cytokines (molecules that trigger immune responses) in mouse models. This indicates that the STEM-engineered cells could be safer than existing therapies, with a lower risk of dangerous immune reactions. “Toxicity has been a major issue in CAR T immunotherapy, and these substantial reductions in cytokine release could make the therapy safer and more tolerable for patients,” said Wang. The authors added, “The major advantages of ZAP327-driven CAR T cells are reduced proinflammatory cytokine production and enhanced T cell persistence, thus generating robust antitumor immunity without potential toxicity and disease relapse.”
The researchers are also testing the STEM approach with T-cell receptor T cell (TCR-T) therapy, a different type of immunotherapy that is more effective with solid cancers. “Our findings not only provide a deep understanding of how TCR signaling regulates cytokine production, tumor killing, metabolic pathway switch, and TSCM cells but also spur the development of the next generation of CAR T cell therapy with improved safety and long-lasting antitumor immunity, which may be also applicable to TCR T cell immunotherapy,” they stated.
Following their encouraging early results, the research team will pursue a clinical trial that tests STEM-engineered CAR T cells in patients. They are also working to develop CAR T cells that can recognize and target more than one protein on cancer cells, making it easier to detect and distinguish them from healthy cells.