In a groundbreaking advancement within the realm of oncology, researchers have unveiled a highly promising non-invasive nomogram designed to predict the prognosis of hepatocellular carcinoma (HCC) patients with unprecedented accuracy. This innovative model leverages the novel biomarker Interleukin-41 (IL-41), marking a transformative step in the clinical management of liver cancer. Hepatocellular carcinoma remains one of the most prevalent and deadly forms of liver malignancy globally, with prognosis often complicated by tumor recurrence and mortality following surgical interventions. The development of predictive tools such as this nomogram offers potential not only to refine patient stratification but also to tailor therapeutic strategies more effectively.
The study, conducted by Mu et al., enrolled a cohort of 224 HCC patients who underwent R0 resection, a procedure ensuring the complete removal of visible tumor tissue with clear margins. The patient population was methodically divided into a training cohort comprising 149 individuals and a validation cohort of 75 patients to ensure the robustness and reproducibility of the findings. By analyzing this patient data, the researchers sought to elucidate the key independent risk factors influencing both tumor recurrence and mortality post-resection. Their approach incorporated comprehensive clinical, pathological, and biochemical parameters, with a special focus on the expression levels of IL-41 within tumor tissues.
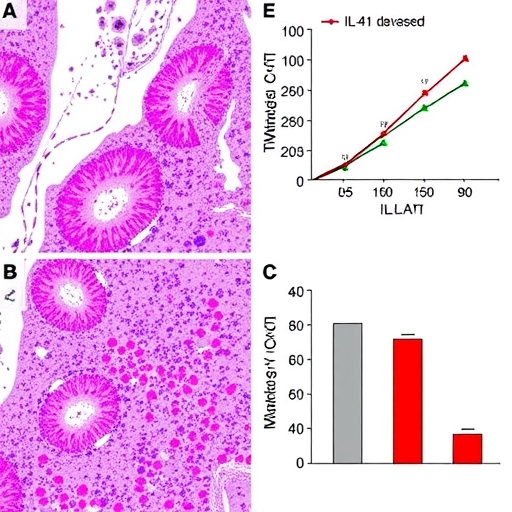
The recurrence of HCC remains a formidable challenge, occurring in nearly half of the patients after surgery. In this investigation, the overall recurrence rate was determined to be 43.3%, underscoring the urgent need for predictive markers. The multivariate Cox regression analysis revealed that elevated intratumoral expression of IL-41 significantly associates with an increased risk of postoperative tumor recurrence, exhibiting a hazard ratio (HR) of 2.446. This finding suggests that IL-41 plays a critical role in the tumor microenvironment, potentially modulating tumor aggression and metastatic potential. Additionally, factors such as the presence of intratumoral arterial structures and microvascular invasion (specifically the MVI1 subgroup) were also identified as independent contributors to recurrence risk.
Beyond recurrence, mortality remains a linchpin in assessing patient outcomes after HCC surgery. The study reported a mortality rate of 15.63% within the follow-up period, a statistic that poignantly reflects the aggressive nature of the disease and its systemic impact. High IL-41 expression stood out again as a potent prognostic marker for poor survival, with a hazard ratio of 4.679, indicating a more than fourfold increased risk of death. Tumor size exceeding five centimeters and elevated levels of Aspartate transaminase (AST) within the 45–90 U/L range were further implicated as mortality risk factors. AST, a liver enzyme indicative of hepatic injury or dysfunction, highlights the interconnectedness of tumor biology and systemic liver impairment.
The molecular underpinnings of IL-41’s role in HCC prognosis merit careful exploration. IL-41, a relatively novel cytokine within the interleukin family, is emerging as a potential modulator of immune responses within the tumor milieu. Its overexpression may facilitate tumor promotion by orchestrating inflammatory pathways, angiogenesis, or immune evasion, although definitive mechanisms require further elucidation. This cytokine’s capacity to serve as a biomarker opens avenues for both diagnostic refinement and targeted therapies aimed at attenuating its pathological effects.
Construction of the nomogram was carried out by integrating these independent risk predictors, resulting in two distinct predictive models—one for tumor recurrence and another for mortality. The models were then rigorously validated, demonstrating high discriminative power and predictive accuracy. Such nomograms provide clinicians with a practical, quantitative tool to estimate individual patient risk profiles rapidly. By inputting patient-specific parameters, physicians can stratify patients into risk categories, allowing more informed clinical decisions regarding surveillance intensity, adjuvant therapy, and personalized care strategies.
The non-invasive nature of this predictive tool significantly enhances its clinical utility. Unlike invasive biopsy procedures or complex genomic analyses, the nomogram employs readily accessible clinical data and biomarker assessments, facilitating widespread application even in resource-limited settings. This ease of use promises to democratize advanced prognostication and ensures that more patients benefit from precision medicine approaches.
Microvascular invasion (MVI) recognized as a hallmark of aggressive HCC, was classified into subgroups, with the MVI1 grade notably correlating with post-surgical recurrence. The study’s elucidation of MVI substantiates its role as a pathological driver of tumor dissemination through vascular channels, reinforcing its significance in prognosis determination. Furthermore, the presence of an intratumoral artery reflects neovascularization processes that sustain tumor growth and facilitate metastatic spread, serving as a radiological and pathological indicator of malignancy severity.
Aspartate transaminase’s inclusion as an independent mortality risk factor bridges biochemical pathology with oncologic outcomes. Elevated AST levels may reflect underlying chronic liver disease, cirrhosis, or acute hepatic injury, all of which adversely influence postoperative recovery and long-term survival. Thus, integration of liver function metrics within the prognostic model underscores the multifactorial nature of HCC prognosis, where intrinsic tumor biology interplays with host hepatic status.
The study by Mu et al. sets a precedent for incorporating novel immunological markers into predictive oncology. The identification of IL-41 as a robust prognostic determinant invites further research into its biological pathways and therapeutic targeting. As immunotherapy increasingly becomes a cornerstone of cancer treatment, understanding cytokine influences such as IL-41 offers potential synergy with emerging modalities like checkpoint inhibitors and adoptive cellular therapies.
Looking forward, the clinical adoption of these nomograms could revolutionize HCC management, improving patient counseling, optimizing follow-up schedules, and guiding adjuvant treatment decisions. Moreover, the framework established here encourages the integration of additional biomarkers and imaging features to refine predictive models further, tailoring prognostication to evolving therapeutic landscapes.
This research embodies a significant leap toward personalized oncology in hepatology. By bridging molecular insights with practical prognostic tools, it empowers clinicians to confront the daunting challenge of HCC recurrence and mortality with enhanced precision. Ultimately, this paves the way for improved survival outcomes and a better quality of life for patients grappling with this formidable malignancy.
The robustness of the model arises from comprehensive validation in both training and independent cohorts, confirming its reproducibility across diverse patient populations. Such methodological rigor ensures that the nomogram’s predictive capacity is not confined to a single dataset but possesses broad applicability, strengthening its potential for global clinical use.
In conclusion, the introduction of IL-41 as a novel marker combined with established pathological and biochemical risk factors culminates in a sophisticated nomogram capable of accurately predicting poor prognosis in hepatocellular carcinoma. This advancement underscores the vital role of interdisciplinary research, integrating immunology, pathology, and clinical oncology to confront one of the most lethal liver cancers.
Subject of Research: Prognostic prediction in hepatocellular carcinoma using the novel biomarker Interleukin-41.
Article Title: A non-invasive nomogram for the prediction of poor prognosis of hepatocellular carcinoma based on the novel marker Interleukin-41.
Article References:
Mu, Z., Su, J., Yi, J. et al. A non-invasive nomogram for the prediction of poor prognosis of hepatocellular carcinoma based on the novel marker Interleukin-41. BMC Cancer 25, 941 (2025). https://doi.org/10.1186/s12885-025-14344-0
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14344-0
Tags: clinical management of hepatocellular carcinomagroundbreaking research in liver malignancyHepatocellular carcinoma prognosisIL-41 biomarker for liver cancermortality risk factors post liver resectionMu et al. liver cancer studynon-invasive nomogram for HCCnovel biomarkers in oncologypatient stratification in liver cancerpredictive tools for liver cancer treatmenttherapeutic strategies for HCCtumor recurrence prediction in liver cancer