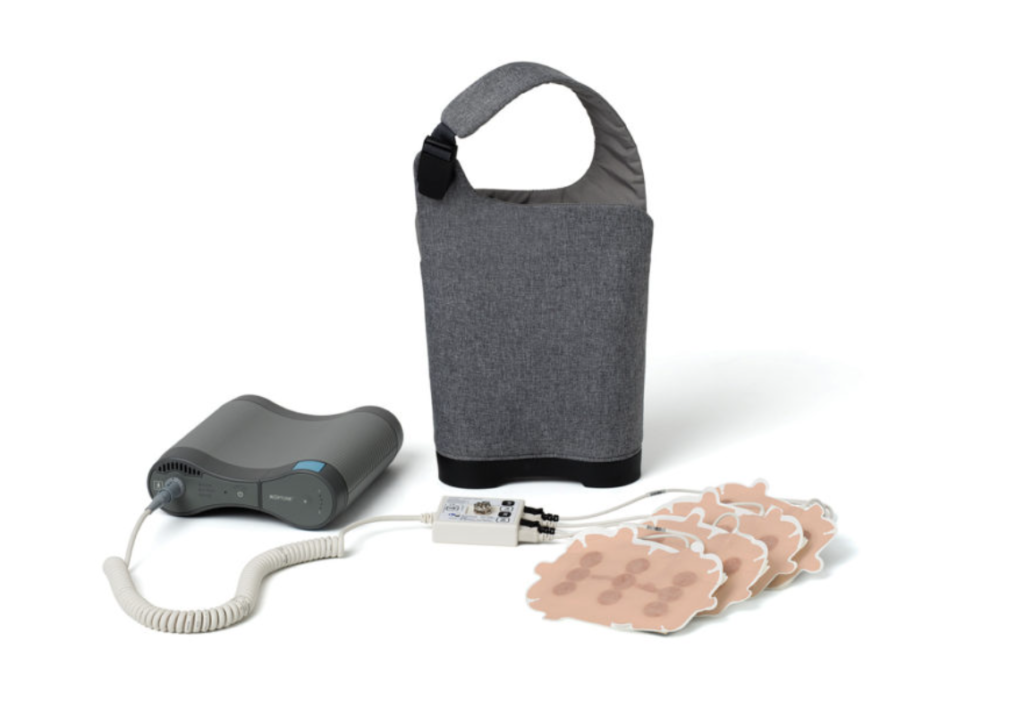
The FDA gave the go-ahead to Novocure for its tumor-treating electric fields device that targets advanced pancreatic cancer in conjunction with the drugs gemcitabine and nab-paclitaxel.
The approval from the U.S. regulatory agency comes a little more than two months after the company released positive phase 3 clinical results of its wearable therapy, dubbed Optune Pax, which showed improved overall survival for what is considered among the most lethal of cancers, the company said in a Feb. 11 press release.
In the study, the device demonstrated a median overall survival of 16.2 months, for a statistically significant improvement over the 14.16 months seen in the control arm.
High-frequency, electric field-based therapies are delivered via pads worn over the skin that physically interfere with the charged structures inside cancer cells as they reproduce, which aids the immune system in responding to the tumor.
Related
“The FDA approval of Optune Pax marks the first new treatment in decades for people living with locally advanced pancreatic cancer,” Frank Leonard, Novocure’s chief executive, said in a statement. “Systemic therapies have shown poor bioavailability in pancreatic tumors, limiting their effectiveness.
“Optune Pax is a fundamentally different treatment, utilizing a biophysical approach that targets the unique electrical properties of cancer cells.”
Pancreatic cancer is known to trigger significant pain as it progresses, making pain management a key challenge.
In the Panova-3 study, time to pain progression was defined as the time from baseline until an increase of 20 or more points was reported by patients on a visual scale for pain or until death. The study found that patients treated with Optune Pax along with gemcitabine and nab-paclitaxel had a median time to pain progression of 15.2 months compared to a median 9.1 months in the group treated with the two drugs along.
The TTFields-powered Optune therapies have also been approved by the FDA for glioblastoma and mesothelioma.