Bone-destructive diseases such as osteoporosis and chronic inflammatory arthritis represent a major global health challenge, affecting millions and leading to pain, fractures, and a severe decline in quality of life. Central to these conditions is the dysregulation of osteoclasts, the specialized cells tasked with the resorption, or breakdown, of bone tissue. Osteoclast overactivity results in an imbalance where bone degradation surpasses bone formation, ultimately driving the progression of debilitating skeletal diseases. Over recent years, research efforts have intensely focused on understanding the molecular pathways that regulate osteoclastogenesis to develop effective therapeutic interventions capable of mitigating pathological bone loss.
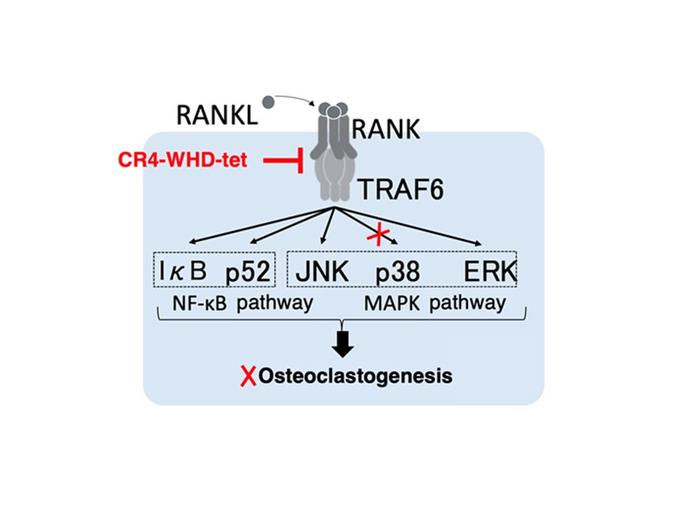
One critical signaling axis that controls the formation and function of osteoclasts is the receptor activator of nuclear factor kappa B ligand (RANKL) and its receptor RANK, which interacts with the intracellular adaptor molecule tumor necrosis factor receptor-associated factor 6 (TRAF6). Upon RANKL’s binding to RANK on osteoclast precursors, TRAF6 is recruited and signals downstream cascades necessary for osteoclast differentiation and activation. This RANK-RANKL-TRAF6 pathway plays a pivotal role in osteoclast biology, making it a prime target for drug development aimed at bone-destructive diseases.
However, the broad physiological roles of the RANKL-RANK-TRAF6 signaling axis impose substantial limitations on therapies that seek to inhibit this pathway globally. Beyond bone metabolism, this signaling network influences immune system function, mammary gland development, and other vital biological processes. Consequently, therapeutics that non-selectively block RANKL-RANK-TRAF6 interactions risk provoking severe off-target effects, including impaired immunity and developmental abnormalities. Additionally, attempts to develop synthetic peptides that disrupt the RANK-TRAF6 interface have historically struggled with poor efficacy, often requiring supraphysiological dosages to exert any meaningful inhibition, thereby compromising their clinical potential.
A groundbreaking study led by Professor Kiyotaka Nishikawa at Doshisha University, Japan, offers a novel paradigm in osteoclast-targeted therapy. Published in Communications Biology, this research introduces a tetravalent peptide named WHD-tet, engineered to modulate the RANK-TRAF6 interaction with unprecedented specificity. Unlike prior peptides, WHD-tet binds TRAF6 multivalently, enabling a high-affinity yet nuanced regulation of downstream signal transduction. More importantly, the researchers developed a cell-permeable derivative, CR4-WHD-tet, which demonstrated potent suppression of osteoclastogenesis in vitro at significantly lower concentrations than any previously reported molecules targeting this pathway.
The mechanism of CR4-WHD-tet’s action is particularly noteworthy. Instead of completely abrogating TRAF6 signaling, the peptide selectively inhibits the recruitment of MKK3, a kinase vital for downstream activation of p38 mitogen-activated protein kinase (p38-MAPK). This subtle modulation effectively prevents the activation of p38-MAPK, a key signaling molecule implicated in terminal osteoclast differentiation and function. By fine-tuning post-receptor signaling events rather than indiscriminately blocking them, CR4-WHD-tet offers a precision approach to osteoclast inhibition, potentially minimizing adverse effects linked to broad-spectrum pathway blockade.
In vivo experiments further validate the therapeutic promise of CR4-WHD-tet. Using mouse models of RANKL-induced bone loss, systemic administration of the peptide significantly attenuated bone resorption, preserving bone density and structural integrity. The peptide’s preferential accumulation in bone tissue is attributed to its high acidic amino acid content, facilitating targeted delivery where osteoclast activity is pathologically elevated. This targeted localization may enhance drug efficacy and reduce systemic toxicity—a major advantage over existing treatments.
Additionally, CR4-WHD-tet’s selective action spares osteoblasts, the bone-forming cells crucial for maintaining skeletal homeostasis. This finding is critical because many anti-osteoclastic agents disrupt the delicate balance between bone resorption and formation, often leading to compromised bone regeneration. By preserving osteoblast viability and functionality, CR4-WHD-tet could foster a more balanced therapeutic outcome, combining inhibition of bone destruction with intact bone formation processes.
Beyond translational implications, this study also illuminates the temporal complexity inherent to osteoclast differentiation signaling pathways. It underscores MKK3’s pivotal function at specific stages of osteoclastogenesis—particularly in controlling the nuclear translocation of p38-MAPK during late differentiation phases. This insight refines current understanding of osteoclast biology, revealing stage-specific molecular targets that can be exploited to design more effective and tailored therapies.
The significance of these findings extends beyond bone disease treatment. The concept of fine-tuning intracellular signaling cascades rather than bluntly inhibiting them heralds a shift in drug design philosophy, emphasizing modulation over suppression. Such an approach may be broadly applicable to other signaling pathways involved in diverse diseases, enabling the development of agents with higher efficacy and fewer side effects.
Professor Nishikawa articulates this perspective, noting that the tetravalent peptide’s selective inhibition mechanism “represents a novel type of therapeutic agent for osteoclast-related diseases that could circumvent the side effects associated with conventional medications.” This pioneering strategy addresses the long-standing challenge of dissecting complex signaling networks to achieve targeted intervention, marking a significant stride in molecular medicine.
As osteoporotic fractures and arthritis-related bone damage continue to impose a heavy burden on healthcare systems worldwide, innovative therapies like CR4-WHD-tet could revolutionize patient management. By safeguarding bone density and minimizing systemic adverse effects, such treatments hold the prospect of dramatically improving patient outcomes and quality of life.
Looking ahead, further preclinical and clinical studies are warranted to assess the safety, efficacy, and pharmacokinetics of CR4-WHD-tet in human populations. Optimization of peptide delivery methods and investigations into long-term effects will be critical to translating this promising molecule into a clinically viable option. Nevertheless, this research lays a robust foundation for next-generation therapeutics that precisely calibrate intracellular signaling to combat bone-destructive diseases.
In summation, the work led by Professor Nishikawa and colleagues redefines the therapeutic landscape for osteoclast-related disorders. By leveraging a tetravalent peptide to precisely modulate RANK-TRAF6-dependent signaling, they demonstrate a novel, effective, and potentially safer approach to controlling pathological bone resorption. This advancement not only enriches scientific understanding of osteoclast biology but also ignites hope for millions suffering from debilitating bone diseases, bringing the vision of fine-tuned molecular therapy closer to reality.
Subject of Research: Animals
Article Title: Clustered peptide regulating the multivalent interaction between RANK and TRAF6 inhibits osteoclastogenesis by fine-tuning signals
News Publication Date: 22-Apr-2025
Web References: http://dx.doi.org/10.1038/s42003-025-08047-2
Image Credits: Professor Kiyotaka Nishikawa from Doshisha University, Japan
Keywords: Health and medicine, Bone diseases
Tags: bone resorption regulationbone tissue healthchronic inflammatory arthritis managementinnovative approaches to bone diseasesmolecular pathways in osteoclastogenesisosteoclast developmentosteoclast differentiation mechanismsosteoporosis treatment strategiesprecision medicine in osteoclast targetingRANK-RANKL-TRAF6 signaling pathwaytargeted therapy for bone diseasestherapeutic interventions for bone loss