A groundbreaking study from Toho University has unveiled a promising new avenue in the early detection of Dementia with Lewy Bodies (DLB), a debilitating neurodegenerative disorder that shares overlapping symptoms with Alzheimer’s and Parkinson’s diseases, often complicating accurate diagnosis. Spearheaded by Associate Professor Ayako Okado-Matsumoto and a multidisciplinary team, this research delves deep into the enigmatic behavior of α-synuclein, a protein intricately linked with neurodegeneration, revealing its altered distribution in blood components of DLB patients.
Alpha-synuclein, a protein predominantly expressed in neural tissue, has long been implicated in the pathogenesis of several neurodegenerative diseases due to its propensity to aggregate into pathological inclusions. In diseases like Parkinson’s and DLB, insoluble α-synuclein aggregates, termed Lewy bodies, accumulate within neurons, a hallmark that disrupts normal cellular functioning. While prior research has extensively focused on brain tissue, the current study shifts its lens to peripheral blood, seeking accessible biomarkers that can reflect central nervous system pathology with minimal invasiveness.
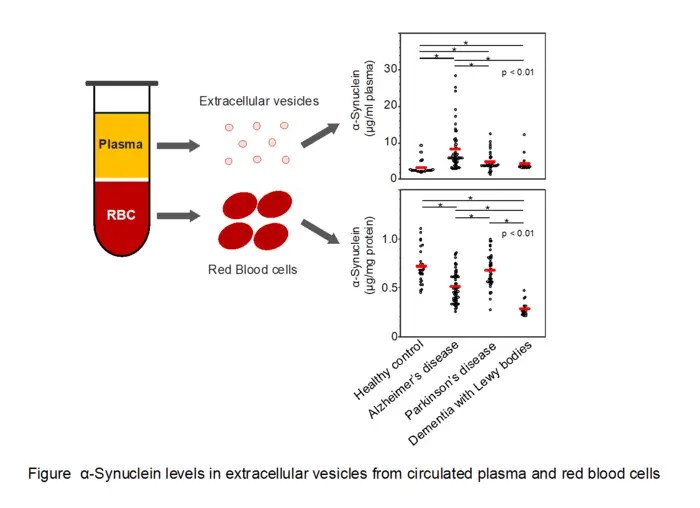
In a meticulously designed observational study, the team analyzed α-synuclein concentrations in both red blood cells (erythrocytes) and plasma-derived extracellular vesicles (EVs) from a broad cohort of individuals, including healthy controls and patients diagnosed with DLB, Alzheimer’s disease, and Parkinson’s disease. Extracellular vesicles, membrane-bound nanostructures secreted by cells, are emerging as critical mediators of intercellular communication and potential reservoirs of disease biomarkers due to their ability to carry proteins, lipids, and nucleic acids reflective of their parent cells.
.adsslot_MxdYswqmaF{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_MxdYswqmaF{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_MxdYswqmaF{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The results revealed a striking reduction in α-synuclein levels within erythrocytes of patients suffering from Dementia with Lewy Bodies, a finding that distinguishes DLB from other neurodegenerative disorders. This depletion in red blood cells stands in contrast to the elevated α-synuclein concentrations found in plasma extracellular vesicles of patients with DLB, as well as those with Alzheimer’s and Parkinson’s disease. The dichotomous distribution pattern hints at a complex pathophysiological mechanism governing α-synuclein trafficking and sequestration.
Further biochemical analyses suggest that the decreased α-synuclein in erythrocytes may reflect systemic alterations in protein handling or clearance mechanisms in DLB, potentially linked to cellular uptake or degradation pathways. Erythrocytes, though anucleate, have been recognized to carry various proteins and contribute to peripheral biomarkers, making this finding particularly compelling for clinical applications involving blood-based diagnostics.
Simultaneously, elevated α-synuclein within plasma EVs across multiple neurodegenerative diseases underscores the role of these vesicles as vehicles of pathological proteins. It is postulated that EVs may facilitate the spread of α-synuclein aggregates between cells, propagating neurodegeneration in a prion-like fashion. Quantifying α-synuclein in vesicle-enriched plasma fractions may therefore serve as a dynamic indicator of disease activity and progression.
The implications of these discoveries extend far beyond biomarker identification. The facile accessibility of blood components for analysis contrasts sharply with the invasive, costly, and time-consuming nature of cerebrospinal fluid sampling or neuroimaging currently used in dementia diagnostics. A robust blood-based α-synuclein test utilizing erythrocyte and plasma EV profiles could revolutionize early detection paradigms, allowing clinicians to differentiate DLB with higher specificity and at earlier stages when therapeutic interventions might be most effective.
DLB affects approximately 1.4 million individuals in the United States alone, yet remains underdiagnosed due to overlapping clinical symptoms and a lack of definitive biomarkers. Early and accurate diagnosis is critical, as treatments and management strategies diverge significantly between DLB and other dementias. By introducing a minimally invasive biomarker grounded in cellular and molecular pathology, this research paves the way for transformative changes in patient care pathways.
The study’s comprehensive methodology included state-of-the-art protein quantification techniques, such as immunoassays calibrated for sensitivity and specificity, alongside rigorous sample processing protocols to isolate erythrocytes and plasma extracellular vesicles. The precision in detecting subtle variations in α-synuclein underscores the potential clinical viability of translating these findings into diagnostic assays suitable for routine medical laboratories.
Moreover, the interdisciplinary collaboration among neurobiologists, clinicians, and biochemists at Toho University and affiliated medical centers represents a model for tackling complex neurodegenerative disorders. Their joint effort has not only highlighted neurobiological nuances but has also contributed to the translational bridge necessary for scientific discoveries to impact bedside diagnostics.
While further longitudinal studies and larger cohorts are warranted to validate and refine these biomarkers’ predictive power and reproducibility, the current evidence robustly supports α-synuclein profiling in blood as a promising biomarker for DLB. Integration with clinical criteria and possibly other molecular markers may enhance diagnostic accuracy and inform personalized therapeutic approaches.
Looking forward, this pioneering work invites expanded investigations into the functional roles of erythrocytes and extracellular vesicles in neurodegeneration. Deciphering the mechanisms underlying altered α-synuclein distribution could reveal novel therapeutic targets to halt or slow the progression of DLB and related diseases. Additionally, exploring the interplay between central pathology and peripheral manifestations may illuminate systemic aspects of neurodegeneration previously overlooked.
In summary, the identification of reduced erythrocytic α-synuclein coupled with elevated plasma extracellular vesicle α-synuclein provides a dual biomarker axis with significant potential for distinguishing Dementia with Lewy Bodies from other neurological dementias. This breakthrough could revolutionize current diagnostic strategies, offering hope for earlier interventions and improved quality of life for millions affected worldwide.
Subject of Research: People
Article Title: The potential of erythrocyte α-synuclein to differentiate dementia with Lewy bodies from Parkinson’s and Alzheimer’s diseases
News Publication Date: April 16, 2025
Web References: 10.1093/jb/mvaf017
References: Ryosuke Amagai, Ryunosuke Hosoi, Sakura Yoshioka, Taiki Maruyama, Takayuki Kawai, Soroku Yagihashi, Hitoshi Nukada, Ryuji Sakakibara, Ayako Okado-Matsumoto. The Journal of Biochemistry, April 16, 2025.
Image Credits: Ayako Okado-Matsumoto
Keywords: α-synuclein, dementia with Lewy bodies, extracellular vesicles, erythrocytes, blood biomarker, neurodegeneration, Parkinson’s disease, Alzheimer’s disease, early diagnosis, neurobiology, protein aggregation, translational medicine
Tags: Alzheimer’s disease comparisonDementia with Lewy Bodies early detectionextracellular vesicles in neurodegenerative disordersmultidisciplinary approach to dementia researchneurodegenerative disease biomarkersnon-invasive diagnostic methods for DLBParkinson’s disease symptoms overlappathological inclusions in neurodegenerationperipheral blood analysis in dementiared blood cells in DLB patientsToho University research studyα-synuclein levels in blood