In a groundbreaking advancement at the intersection of oncology and artificial intelligence, researchers have developed a sophisticated machine learning framework capable of delivering remarkably precise survival predictions for patients diagnosed with prostate adenocarcinoma. This malignancy, the predominant form of prostate cancer, poses significant clinical challenges due to its heterogeneous nature and complex progression patterns. Utilizing an assembly of ensemble learning techniques, the novel approach signifies a transformative step toward integrating computational intelligence into oncological prognostics.
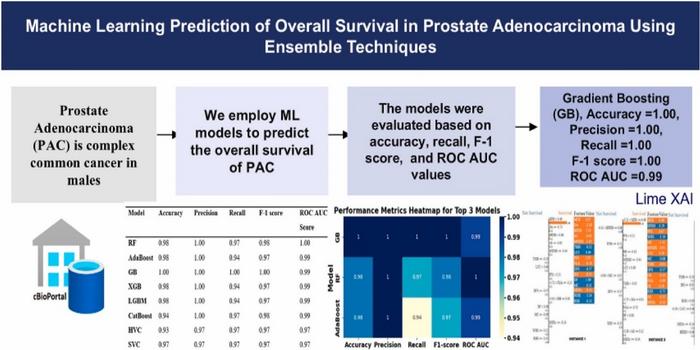
Prostate adenocarcinoma represents the vast majority of prostate cancer cases, making early and accurate survival estimation critical for effective patient management. The research team, comprising experts from the University of Sharjah in the United Arab Emirates and Near East University in Turkey, harnessed eight distinct ensemble machine learning models to analyze patient data rigorously. These models—Random Forest (RF), AdaBoost, Gradient Boosting (GB), Extreme Gradient Boosting (XGB), LightGBM (LGBM), CatBoost, Hard Voting Classifier (HVC), and Support Vector Classifier (SVC)—were systematically evaluated to determine their predictive capacities concerning overall survival outcomes.
The data foundation for this study was extracted from The Cancer Genome Atlas (TCGA) PanCancer Atlas, a comprehensive repository containing molecular and clinical information on diverse cancer types. This dataset permitted researchers to rigorously train and validate their machine learning models, emphasizing robustness and clinical applicability. Performance metrics such as accuracy, precision, recall, F1-score, and the ROC-AUC score served as the critical evaluative indicators to quantify each algorithm’s effectiveness in forecasting patient survival.
.adsslot_YkVrKpFizX{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_YkVrKpFizX{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_YkVrKpFizX{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Among the tested methodologies, Gradient Boosting emerged as the unequivocal frontrunner, attaining near-perfect scores across all performance parameters. The GB model achieved a flawless 1.0 in accuracy, precision, recall, and F1-score, alongside a commendable 0.99 in ROC-AUC. This impeccable performance underscores GB’s superior ability to classify true positive cases while minimizing false negatives—an essential feature in predictive oncology where misclassification can lead to adverse clinical consequences.
Other ensemble techniques, notably Random Forest and AdaBoost, demonstrated substantial predictive prowess as well. Random Forest’s interpretability and robustness allowed it to effectively discriminate between patients with divergent survival prospects. AdaBoost, known for its iterative focus on misclassified instances, further reinforced the predictive landscape by optimizing model sensitivity. The complementary strengths of these models highlight the value of ensemble strategies in addressing the multifaceted challenge of survival prediction in prostate cancer.
The clinical significance of these findings cannot be overstated. Prostate adenocarcinoma remains one of the most lethal cancers affecting men worldwide, second only to skin cancer in incidence rates. The disease predominantly arises from glandular cells within the prostate, a walnut-sized organ situated below the urinary bladder and anterior to the rectum. With over three million men diagnosed in the United States alone and a mortality rate of approximately one in 44 diagnosed patients, improving prognostic accuracy has become a paramount medical imperative.
Early detection and precise survival prognostication can dramatically improve treatment outcomes, guiding therapeutic decisions and personalized care strategies. Traditional diagnostic markers and clinical assessment tools have historically faced limitations due to the prostate cancer’s heterogeneous presentation and frequent comorbid conditions in affected patients. This complexity has driven the quest for more sophisticated, data-driven predictive techniques capable of navigating such clinical intricacies.
The incorporation of ensemble machine learning models into clinical workflows presents a promising avenue to surmount these obstacles. As co-author Dr. Dilber Ozsahin of the University of Sharjah emphasizes, integrating Gradient Boosting and related ensemble methods into routine diagnostics offers urologists and oncologists a potent adjunct for decision-making. By reliably predicting overall survival, these models empower clinicians to tailor treatment modalities with heightened confidence, potentially improving patient prognosis and quality of life.
Beyond immediate clinical application, the predictive model introduced by the research team exemplifies how computational intelligence can simulate complex biological phenomena. The study’s computational simulation and modeling approach harnesses the iterative learning capabilities of ensemble algorithms to resolve nonlinear associations within genomic and clinical data, thereby echoing the intricate interplay of genetic, environmental, and lifestyle factors influencing cancer progression.
The researchers highlight the necessity of expanding these initial findings through validation on larger and more diverse datasets. Incorporating additional variables, such as patient lifestyle factors, emerging biomarkers, and longitudinal health records, could further refine model accuracy and applicability across heterogeneous clinical populations. Such enhancements would bolster the transition of ensemble learning-based prognostics from theoretical constructs to indispensable clinical tools.
While the current study’s results are promising, the researchers remain cautious, underscoring the importance of conducting prospective clinical trials to assess real-world efficacy. The adaptation of advanced AI models into healthcare demands rigorous evaluation to ensure generalizability, ethical integrity, and patient safety. The deployment of ensemble machine learning techniques hence represents an evolving frontier poised to redefine prognostic paradigms in oncology.
In summary, this innovative research demonstrates that ensemble machine learning models—particularly Gradient Boosting—can achieve exceptional predictive accuracy for overall survival in prostate adenocarcinoma patients. By leveraging comprehensive genomic and clinical datasets, the study paves the way for AI-powered prognostic tools that could transform prostate cancer management. As healthcare increasingly embraces artificial intelligence, such studies exemplify how data science can yield tangible clinical benefits, fostering personalized medicine and improved patient outcomes.
Subject of Research: Not applicable
Article Title: Machine learning prediction of overall survival in prostate adenocarcinoma using ensemble techniques
News Publication Date: 1-May-2025
Web References: 10.1016/j.compbiomed.2025.110008
Image Credits: Computers in Biology and Medicine
Keywords: Computer science, applied sciences and engineering
Tags: advanced statistical methods in oncologyAI survival prediction for prostate cancercancer patient management strategiesclinical applications of AI in medicinecomputational intelligence in healthcareensemble learning techniques in cancer researchinterdisciplinary research in cancer treatmentmachine learning in oncologyprecision medicine for prostate cancerpredictive modeling for cancer prognosisprostate adenocarcinoma survival ratesThe Cancer Genome Atlas data analysis