A groundbreaking review published in the journal Genes & Diseases has unveiled transformative insights into the multifaceted role of LINE-1 (Long Interspersed Nuclear Element-1) retrotransposons in preimplantation development and the maintenance of totipotency in mammalian embryos. For decades, LINE-1 elements were largely dismissed as dormant genomic parasites or evolutionary fossils, but recent research now categorically positions them as central regulators of early embryogenesis, chromatin architecture, and cellular fate decisions. This paradigm shift in understanding LINE-1’s biological function is poised to influence diverse fields, from developmental biology to regenerative medicine and age-related disease research.
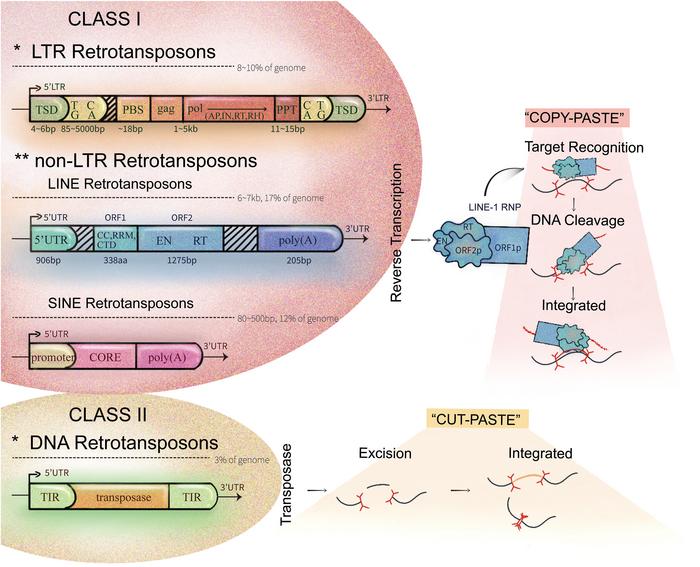
LINE-1 elements are autonomous retrotransposons capable of copying and inserting themselves into new genomic locations through an RNA intermediate. Their enzymatic machinery, primarily mediated by the ORF2 protein with endonuclease and reverse transcriptase activities, initiates target-primed reverse transcription in the genome. This mechanism not only enables genomic plasticity but is intricately intertwined with early embryonic events. The review highlights how, immediately after fertilization, in the zygote, LINE-1 transcripts are actively produced and their proteins expressed, marking the onset of a complex interaction between LINE-1 activity and zygotic genome activation (ZGA). ZGA represents a critical window wherein the embryo shifts from dependence on maternally deposited transcripts to self-sufficiency in gene expression, establishing the foundations of totipotent cellular states.
At a molecular level, LINE-1’s engagement in remodeling chromatin is characterized by the establishment of an open, permissive chromatin landscape conducive to transcriptional activation. The transient yet robust expression of LINE-1 RNA and protein during the early cleavage stages promotes chromatin decondensation and accessibility. Failure to initiate or sustain LINE-1 activity at this juncture correlates with developmental arrest and failure of embryos to progress beyond early cleavage, underscoring LINE-1’s essentiality in embryogenesis. The review delves deep into the biophysical interplay between LINE-1 ribonucleoprotein complexes and chromatin remodelers, suggesting that LINE-1 functions beyond mere transposition, acting as a scaffold for the recruitment of epigenetic modulators.
.adsslot_gKd9R0Hh6x{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_gKd9R0Hh6x{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_gKd9R0Hh6x{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
A significant revelation from recent studies focuses on the crosstalk between LINE-1 and epigenetic pathways. LINE-1 expression precisely influences DNA methylation dynamics, histone post-translational modifications, and RNA methylations such as N6-methyladenosine (m6A), collectively shaping the epigenomic landscape. This multifaceted regulation is critical to maintaining genomic stability while preserving the totipotent state. The review emphasizes that contrary to earlier beliefs of LINE-1 activity being deleterious, controlled expression contributes to a tightly regulated balance between self-renewal and differentiation, correlating with lineage commitment during embryonic development.
Beyond embryogenesis, LINE-1’s influence extends into stem cell biology. Its expression patterns and regulatory nuances are mirrored in embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), where modulation of LINE-1 impacts stemness and differentiation potential. This connection introduces compelling possibilities for manipulating LINE-1 in cellular reprogramming protocols, potentially enhancing the efficiency and fidelity of iPSC generation. Furthermore, aberrant LINE-1 activity has been implicated in genomic instability characteristic of aging tissues, linking retrotransposon dysregulation with cellular senescence and degenerative diseases.
The review meticulously discusses the molecular safeguards that regulate LINE-1 elements, ranging from cytosine DNA methylation to histone methylation at repressive marks (e.g., H3K9me3), and the contribution of piRNA pathways in germ cells. Such multilayered control ensures LINE-1’s activation is temporally and spatially restricted, preventing uncontrolled retrotransposition that could compromise genomic integrity. Intriguingly, the reactivation of LINE-1 seems to be a deliberate developmental strategy, serving as a genomic “switch” during early embryogenesis, whereas its silencing becomes paramount as cells transition toward lineage specification.
Technological advances such as single-cell RNA sequencing and chromatin accessibility assays (ATAC-seq) have provided unprecedented resolution in characterizing LINE-1 expression dynamics and its impact on the embryonic transcriptome. Computational analyses reveal that LINE-1 transcripts act as non-coding RNA regulators, interacting with chromatin modifiers and transcription factors to orchestrate gene networks underpinning totipotency. Moreover, the identification of novel ORF1p and ORF2p interacting partners advances our understanding of the molecular complexes formed during retrotransposition and their non-canonical roles.
In the context of regenerative medicine, the insights into LINE-1’s role open new avenues for therapeutic intervention. By harnessing or modulating LINE-1 activity, scientists may improve stem cell therapies, enhance tissue regeneration, and possibly counteract the deleterious effects of aging at the molecular level. However, these applications necessitate an intricate understanding of LINE-1 regulation to avoid potential risks associated with genomic insertions and mutagenesis.
Collectively, this comprehensive review positions LINE-1 as a key molecular player in early mammalian development, bridging gaps between genomic plasticity, epigenetic regulation, and cellular identity. As the field moves forward, integrating LINE-1 biology into developmental paradigms promises to deepen our grasp of mammalian development and fuel innovations in biotechnology and medicine.
The implications of these findings resonate beyond basic science, as they provide foundational knowledge to tackle age-associated diseases, cancer genetics, and developmental disorders rooted in epigenetic and genomic dysregulation. Future research into LINE-1 and its regulatory networks is expected to not only elucidate the intricate dance of genome dynamics during the earliest life stages but also pave the way for groundbreaking clinical applications.
Subject of Research: Role of LINE-1 retrotransposons in preimplantation development, totipotency, and cellular reprogramming.
Article Title: Expression of LINE-1 elements is required for preimplantation development and totipotency.
News Publication Date: 2025
Web References: DOI link
References: Ru Ma, Nan Xiao, Na Liu, Genes & Diseases, Volume 12, Issue 5, 2025, Article 101555.
Image Credits: Genes & Diseases
Keywords: LINE-1, retrotransposon, zygotic genome activation, totipotency, preimplantation development, epigenetic regulation, embryonic stem cells, induced pluripotent stem cells, chromatin remodeling, cellular senescence, genomic instability, regenerative medicine
Tags: age-related disease researchcellular fate decisionschromatin architecturedevelopmental biology insightsearly embryo developmentembryonic gene regulationevolutionary role of LINE-1genomic plasticity mechanismsLINE-1 retrotransposonsregenerative medicine applicationstotipotency in mammalszygotic genome activation