In a groundbreaking study set to reshape the landscape of neurological therapeutics, researchers have unveiled the intricate mechanisms behind the safe and reversible opening of the blood-brain barrier (BBB) using focused ultrasound technology. Published in the forward-looking journal Communications Engineering, this investigation dives deep into the transient reorganization of tight junctions—the critical gatekeepers of the BBB. This finding not only cements focused ultrasound as a prime candidate for non-invasive brain drug delivery but also bridges a vital knowledge gap in the neuroengineering field.
The blood-brain barrier is a highly selective and protective interface that prevents harmful substances while maintaining brain homeostasis. Due to its selective permeability, delivering therapeutic agents to targeted brain regions has remained a formidable challenge in treating neurological disorders such as Alzheimer’s, Parkinson’s, brain tumors, and epilepsy. Traditional drug delivery techniques often fail to cross the BBB safely and effectively, limiting the success of many prospective treatments. Focused ultrasound, combined with microbubbles, has emerged as a minimally invasive technique that transiently opens the BBB to allow drugs to reach affected tissues without damaging the brain.
At the heart of the research conducted by Noel, Kugelman, Karakatsani, and colleagues is an exploration of the precise molecular events that enable tight junctions to transiently disengage during focused ultrasound treatment. Tight junctions are complexes of proteins that seal endothelial cells lining the brain’s blood vessels, thereby regulating permeability. Prior to this study, the underlying dynamics facilitating BBB disruption remained poorly understood, raising concerns about safety and efficacy.
Using advanced imaging techniques and molecular assays, the researchers demonstrated that the application of pulsed focused ultrasound induces a rapid yet reversible reconfiguration of tight junction proteins. Instead of causing permanent damage or outright destruction, the ultrasounds trigger a controlled reorganization allowing paracellular pathways to momentarily widen. During this brief window, therapeutic agents circulating in the blood can penetrate into the brain parenchyma, achieving targeted delivery without eliciting inflammation or edema.
Importantly, the study highlights that the blood-brain barrier’s restoration is robust and timely. Within hours post-ultrasound application, tight junctions reassemble, re-establishing the barrier’s integrity and safeguarding the central nervous system from potential toxins. This resilience addresses one of the foremost concerns related to ultrasound-mediated BBB opening—that a compromised barrier could lead to brain infections or neurotoxic exposure over extended periods. The transient nature of junctional reorganization thus solidifies the safety profile of this emerging technique.
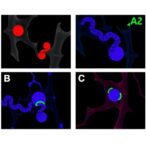
The methodology incorporated in vivo experiments on animal models, where focused ultrasound was applied to hippocampal and cortical regions—key targets for many neurodegenerative and psychiatric conditions. Real-time monitoring of tight junction proteins such as claudins, occludins, and zonula occludens clearly identified spatial-temporal changes corresponding with ultrasound exposure. The researchers noted that the manipulation of ultrasound parameters like frequency, intensity, and exposure duration finely tuned the extent of BBB opening, indicating a controllable therapeutic window.
This study’s implications reverberate well beyond drug delivery. It opens avenues into precision neuromodulation, where localized opening of the BBB might enable selective modulation of brain circuits through bioactive molecules or immune factors. Moreover, the controlled and safe disruption of tight junctions could enhance the efficacy of gene therapy vectors and nanomedicine targeted at brain diseases previously deemed inaccessible.
The discoveries also spark a shift in our understanding of endothelial biology within the central nervous system. Traditionally seen as a static barrier, the BBB is now being appreciated as a dynamic interface capable of finely regulated responses to mechanical stimuli. The researchers propose that the transient reorganization of tight junctions represents a hitherto underappreciated physiological mechanism that might play roles in other contexts like neuroinflammation and repair.
While the outcomes are promising, the study also calls for further investigations into long-term effects, optimized ultrasound regimens, and translation to human clinical applications. The barrier’s complex architecture and the brain’s sensitivity necessitate detailed safety assessments across diverse conditions and patient demographics. Nonetheless, the authors stress that the mechanistic clarity gained forms a solid foundation for clinical trials and eventual widespread therapeutic use.
As we move toward a future where neurodegenerative diseases and brain tumors might be tackled with non-invasive precision interventions, the findings from Noel and colleagues stand as a beacon of innovation. The ability to open the blood-brain barrier safely and transiently via focused ultrasound without collateral damage offers hope for millions worldwide who suffer from disorders once considered untreatable.
In essence, this pioneering research resurrects the notion that the skies above the brain are no longer an impenetrable fortress, but a gatekeeper that can be respectfully enlisted in the fight against neurological disease. The synchronization of engineering precision with molecular biology heralds a new paradigm in therapeutic delivery, acting as a catalyst for advancements in neurotechnology and personalized medicine.
Taking into account the escalating burden of brain disorders globally, techniques that enhance targeted drug delivery while mitigating risks represent critical breakthroughs. The insights into tight junction dynamics add a vital piece to the puzzle, addressing mechanistic uncertainty that has long hindered acceptance of ultrasound-mediated BBB disruption in clinical practice.
Ultimately, this study is more than a technical feat; it is a blueprint for integrating physical modalities and biological responses in healthcare. By harnessing the reversible permeability changes of endothelial junctions, future treatments could be both more effective and less invasive, dramatically improving patient outcomes and quality of life.
Excitingly, ongoing collaborative efforts combining neuroimaging, biomedical engineering, and molecular neuroscience are already building on this research, promising to refine protocols, scale up technology, and expedite regulatory approval. This interdisciplinary synergy exemplifies the future of translational medicine—where tomorrow’s therapies arise from today’s curiosity-driven innovation.
In conclusion, the detailed elucidation of how safe focused ultrasound mediates blood-brain barrier opening through transient tight junction reorganization adds a crucial chapter to neurotherapeutic development. It empowers clinicians and bioengineers alike to strategize smarter, safer brain-targeting interventions, steering the field closer to defeating some of humanity’s most daunting neurological challenges.
Subject of Research: Safe and transient opening of the blood-brain barrier via focused ultrasound, focusing on tight junction reorganization mechanisms.
Article Title: Safe focused ultrasound-mediated blood-brain barrier opening is driven primarily by transient reorganization of tight junctions.
Article References: Noel, R.L., Kugelman, T., Karakatsani, M.E. et al. Safe focused ultrasound-mediated blood-brain barrier opening is driven primarily by transient reorganization of tight junctions. Commun Eng (2026). https://doi.org/10.1038/s44172-026-00597-5
Image Credits: AI Generated
Tags: Alzheimer’s disease ultrasound treatmentblood-brain barrier permeability modulationbrain tumor focused ultrasound therapyfocused ultrasound blood-brain barrier openingmicrobubble-assisted ultrasound therapyneuroengineering drug delivery innovationsneurological disorder treatment methodsnon-invasive brain drug deliveryParkinson’s disease drug deliveryreversible blood-brain barrier disruptiontargeted brain region therapeuticstight junction reorganization