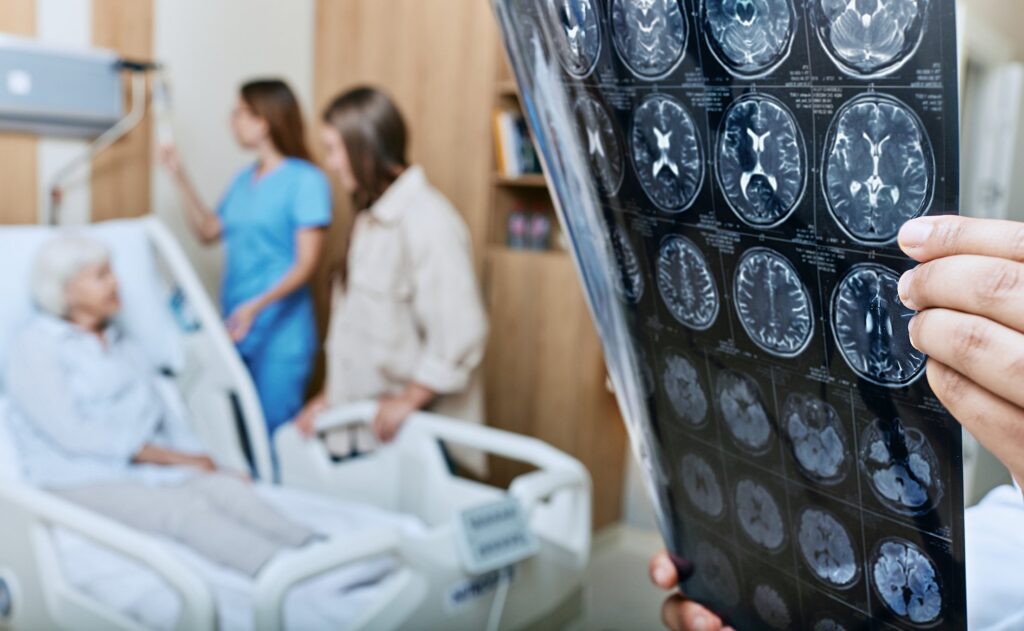
Newly FDA-cleared blood tests are now making it easier for doctors to diagnose Alzheimer’s disease. But could a single blood test also predict when patients’ symptoms will appear?
A study published Feb. 19 in Nature Medicine suggests the answer is yes.
The study, led by the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium, looked at the levels of p-tau217, a protein linked to Alzheimer’s, in blood samples from more than 600 adults ages 62 to 78.
The samples were collected over a period of up to 10 years, and the individuals tested initially were not experiencing cognitive symptoms.
Researchers then built statistical clock models that related changes in the protein levels over time to future symptom onset.
“This model was able to estimate how many years away a person might be from developing memory and thinking problems related to Alzheimer’s with an accuracy of within three to four years,” the FNIH said in a statement.
“This level of accuracy could prove especially helpful for clinical trials, helping researchers select participants mostly likely to develop symptoms within a trial’s timeframe.”
The team also developed an interactive, web-based tool allowing scientists to visualize how levels of p-tau217 change over time and how they relate to Alzheimer’s symptoms, the FNIH said.
Based on the clock models, the team found those with higher levels of p-tau217 tended to develop Alzheimer’s symptoms sooner. Notably, older individuals progressed much faster after reaching elevated levels of the protein, the researchers found. For example, someone who becomes p-tau217-positive at 80 might develop symptoms in roughly 11.4 years, while it may take 20 years for symptoms to show up in someone who first becomes positive at age 60, according to the study.
The researchers said they are working to improve the accuracy of the clock models, which could eventually allow the blood test to be used with patients to inform early treatment decisions.
“With advances in Alzheimer’s blood-based diagnostics, like this study, the field is moving closer to earlier diagnosis and more accessible, precise treatments for people living with the disease,” Alessio Travaglia, Ph.D., director of translational science, neuroscience and rare diseases at the FNIH, said in the statement.
Private sector funders of the study included AbbVie, Biogen, Janssen Research & Development and Takeda, along with the Alzheimer’s Association and the Diagnostics Accelerator at the Alzheimer’s Drug Discovery Foundation.