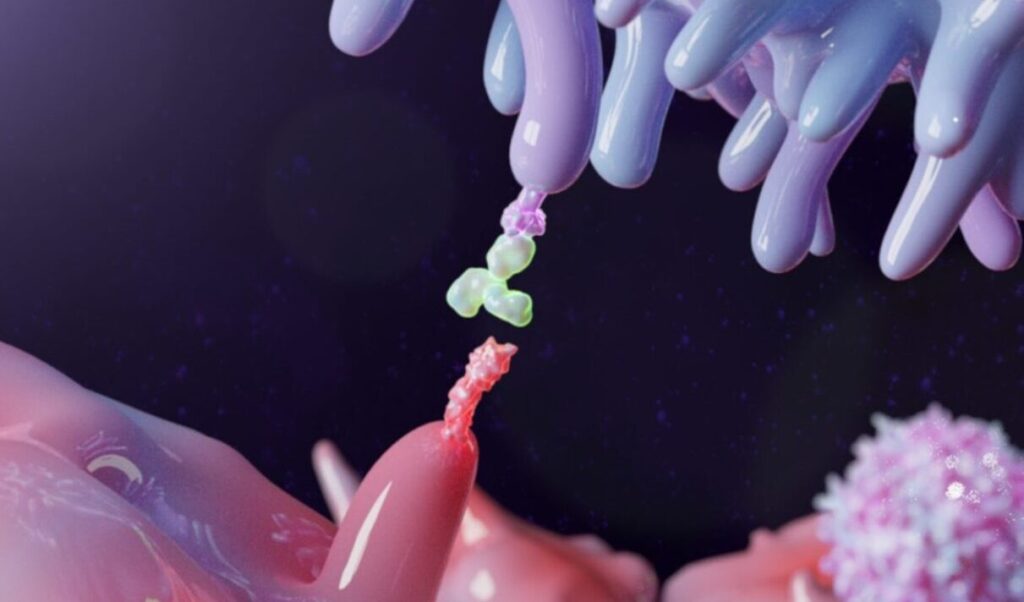
In the next few years, atezolizumab will go off patent in the United States and Europe. This monoclonal antibody is a checkpoint inhibitor that allows a person’s immune system to kill cancer cells. To pave a pathway to producing a biosimilar version of atezolizumab, Mehmet Inan, PhD, director of the Izmir Biomedicine and Genome Center in Turkey, and his colleagues developed a bioprocess for producing this drug in recombinant cells created from the Chinese hamster ovary (CHO) DG44 cell line, which is often used by the pharmaceutical industry.
According to Inan’s team: “There is no documented production process of atezolizumab biosimilar in the literature except one in the plant platform.” So, this team of scientists developed a method for manufacturing a biosimilar of atezolizumab in mammalian cells.
First, Inan and his colleagues used different methods of transfecting CHO DG44 cells with viral genes that would produce atezolizumab. By screening the resulting cell lines, the scientists selected one that produced the most atezolizumab.
Using a batch-fed bioreactor and testing eight chemically defined media that are commercially available, plus supplements, Inan’s team selected the most productive conditions for culturing the cells. From this, the process produced yields of the antibody as high as 800 mg/L.
After a purification process composed of three forms of chromatography—affinity, cation exchange, and ion-exchange—Inan and his colleagues compared the performance of the biosimilar to the commercial form of atezolizumab. The results showed that both forms of atezolizumab produced similar binding of the intended target, which was the programmed death-ligand 1 (PD-L1).
To move this biosimilar into clinical trials, though, industrial scientists would need more thorough testing, including toxicology studies. As this example shows, a long path lies between any approved biological drug and creating a biosimilar that can be used in patients.