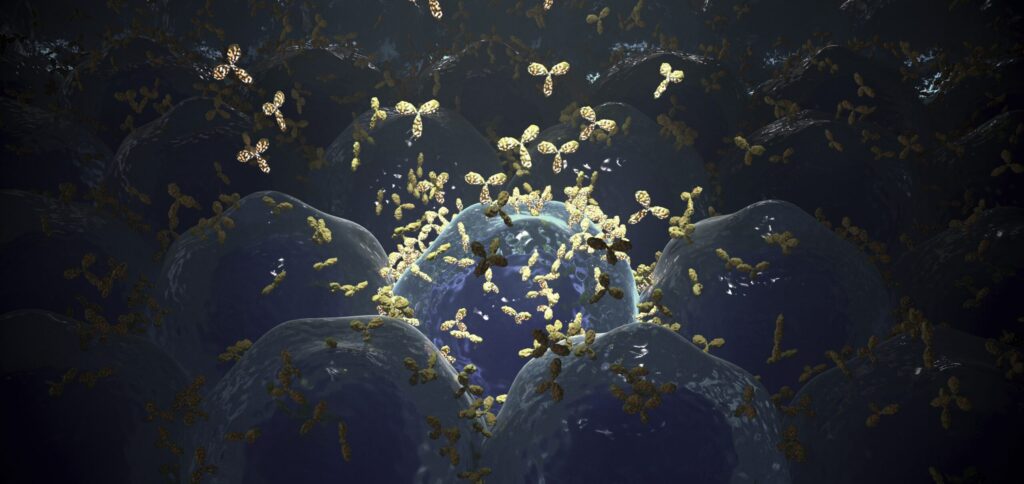
Therapeutic monoclonal antibodies (mAbs) are used in chronic lymphocytic leukemia immunotherapies to target the receptor CD20 at the plasma membrane of immunological B cells. Although they have been in clinical use for several years, the molecular binding mechanisms of mAbs to endogenous CD20 are unclear, as is how antibody binding activates the immune system to kill B cells. Now, researchers from the Biocentre of Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, report they have developed a new super-resolution microscopic method to investigate the interactions of therapeutic antibodies with target molecules on tumor cells in 3D with molecular resolution.
The findings are published in Science in an article titled, “Decoding the molecular interplay of CD20 and therapeutic antibodies with fast volumetric nanoscopy.”
“To decode the molecular interplay of endogenous CD20 on B cells with therapeutic mAbs, three-dimensional (3D) volumetric super-resolution fluorescence imaging methods are needed that provide a high spatiotemporal resolution,” the researchers noted. “To address these limitations, we introduced a speed-optimized variant of DNA-based point accumulation for imaging in nanoscale topography (DNA-PAINT).”
“We can now observe how effectively the antibodies work and thus contribute to the development of improved therapies,” said Markus Sauer, PhD, a professor and and chair of the Department of Biotechnology and Biophysics at JMU.
The new microscopic method is termed LLS-TDI-DNA-PAINT. In their new study, first author Arindam Ghosh, PhD, and a team from Sauer’s chair describe how the newly developed technology works and what findings have already been obtained with it.
The researchers carried out their studies on fixed and living Raji B cells using the new microscopy method. This cell line originates from a patient’s Burkitt’s lymphoma and is often used in cancer research. The researchers brought the cells into contact with one of the four therapeutic antibodies RTX, OFA, OBZ, and 2H7.
All four antibodies crosslink the CD20 molecules in the cell membrane, resulting in strong localized accumulations of the antibodies.
The experiments also show that all four antibodies crosslink CD20 molecules that are located at specific sites on the membrane—on micrometer-long protrusions of the membrane called “microvilli.” At the same time, the binding of the therapeutic antibodies polarizes the B cell and the outstretched microvilli are stabilized. As a result, the B cells take on a kind of hedgehog shape because the membrane protrusions are only located on one side of the cell.
“The previous classification of therapeutic antibodies into types I and II can no longer be maintained,” said Ghosh. Until now, research has assumed that therapeutic antibodies of type I have a different mechanism of action than those of type II. However, the Würzburg studies disprove this.
“The hedgehog shape makes the B cells appear as if they want to form an immunological synapse with another cell,” explained Ghosh. It is conceivable that the treated B cells activate macrophages and natural killer cells of the immune system in this way. The research team now will clarify whether this assumption is correct in further studies.
Their new findings could aid the rational design of improved immunotherapies targeting tumor-associated antigens.