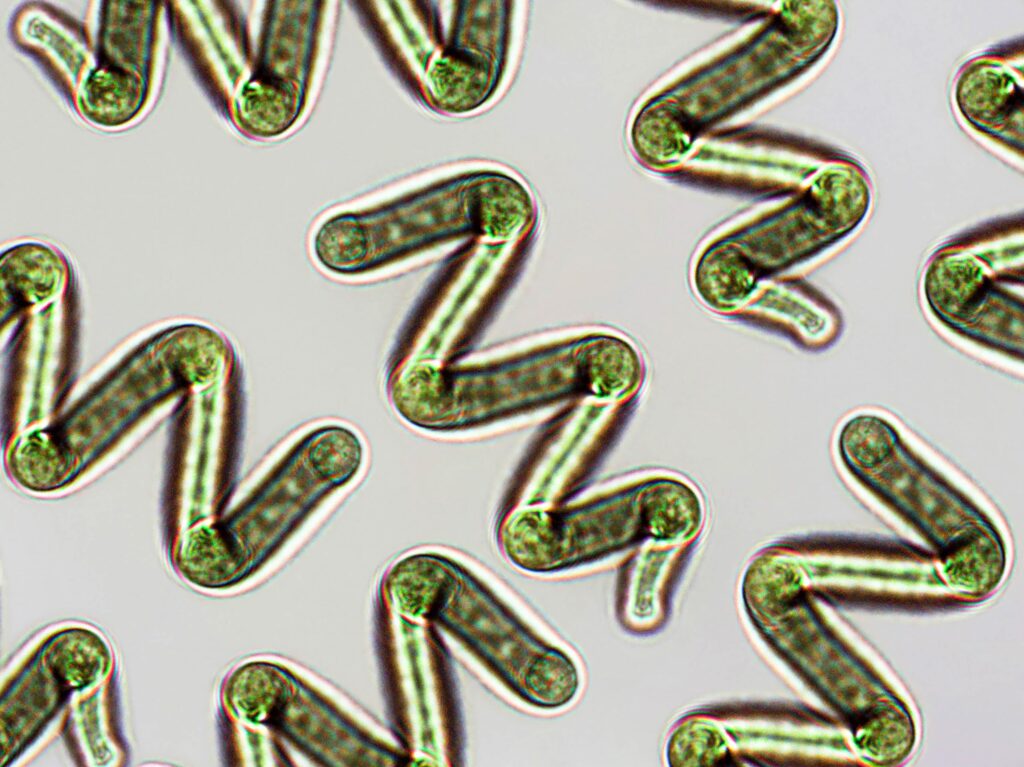
Spirulina has solid potential as a therapeutic adjuvant, according to researchers in Australia who isolated extracellular vesicles (EVs)—nanoscale vesicles enclosed with bilayer phospholipid membranes—from that cyanobacteria and evaluated them in terms of morphology and molecular characterization.
This appears to be the first time researchers have focused specifically on EVs derived from spirulina, although other researchers have evaluated those derived from other cyanobacteria, according to Mohammad Farouq Sharifpour, PhD, postdoctoral research fellow and Alex Loukas, PhD, distinguished professor, both of the James Cook University in Australia and the corresponding authors of a recent paper.
EVs extracted from the Limnospira genus of spirulina have immunomodulatory properties that make them attractive adjuvants for subunit vaccines, the scientists report. These spirulina-derived EVs (SPEVs) also trigger pro-inflammatory responses to innate immune cells without inducing toxicity in mice.
Those attributes may make SPEVs particularly beneficial, especially when the spirulina is genetically modified to express particular therapeutic or immunogenic properties on their surface, Sharifpour suggests. Additional advantages for biopharmaceutical developers include spirulina’s ability to express transgenes at up to 30% of its biomass, and low-cost, large-scale cultivation and isolation.
To efficiently isolate the EVs from the spirulina culture, Sharifpour, Loukas, and colleagues used a combination of low-velocity centrifugations, filtration, ultracentrifugation, and size exclusion chromatography. However, with recent technological advances in the field, EVs could be easily isolated from large-scale (tens of liters) cultures of spirulina using high-throughput size-exclusion chromatography.
Among the 54 identified proteins in SPEVs associated with immune modulation, SLP (associated with outer membrane stability and metal transport), SLH-domain (believed to contribute to immune modulation), and RTX (which may enhance immune stimulation) were notable. Therefore, when SPEVs were injected into mice, they enhanced antigen-specific IgG responses by more than 100-fold.
The team also annotated sequences using gene ontology terms. It found 30 sequences associated with biological processes (typically carbohydrate transport), 35 with cellular components (usually mapped to the plasma membrane-derived thylakoid membrane), and 27 with molecular function (most often porin activity, which mapped to all six identified porins).
Most of the SPEVs formed outer membrane vesicles (OMVs) “with one distinct, bilayered phospholipid membrane,” although outer-inner membrane vesicles (OIMVs) with two membrane layers, similar to gram-negative bacteria, were also present. The most prevalent sizes were linked to how they were measured, with significant differences between nanoparticle tracking analysis and tunable resistive pulse sensing technology.
How SPEVs’ physical properties may affect their use as vaccine adjuvants is, as yet, undetermined.
“We showed that the pro‐inflammatory properties of SPEVs can be utilized as an adjuvant for subunit vaccines, opening up new avenues of industrial biotechnology that exploit the biotherapeutic properties of this microalgae,” they concluded.
Next, Sharifpour says, they plan to “determine the safety and mechanism of action for immunogenicity of spirulina EVs as an adjuvant using in vivo models and human immune cells, and to developing an engineered SPEV that can act as a vaccine and adjuvant simultaneously.”